![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
28 Cards in this Set
- Front
- Back
|
Molar Mass |
mass of one mole of a substance |
|
|
Formula Mass |
mass of a molecule or compound in amu |
|
|
Mole |
a counting unit equal to 602 billion trillion |
|
|
Avogadro's Number |
602 billion trillion (another name for the mole) |
|
|
Physical change |
change to size, shape or state of matter |
|
|
Chemical change |
breaking chemical bonds so that atoms rearrange to form new bonds |
|
|
Combustion Reaction |
burning in oxygen |
|

|
example of combustion reaction |
|

|
decomposition reaction |
|
|
Decomposition reaction |
when a compound splits apart |
|

|
double displacement (replacement) reaction |
|
|
Double Replacement Reaction (Displacement) |
when two compounds split apart and recombine; two components rearrange |
|
|
Single Replacement Reaction (Displacement) |
when one compound splits apart and bonds with a different element; one component rearranges |
|

|
single replacement (displacement) reaction |
|
|
Synthesis Reaction |
where two things combine to create a compound |
|

|
synthesis reaction |
|
|
Chemical Equation |
an equation that explains the types and amounts of elements, molecules or compounds involved in a chemical reaction |
|
|
Law of Conservation of Mass |
mass is neither created nor destroyed during a chemical reaction |
|
|
Endothermic Reaction |
when compounds take in energy to create bonds to form new products; temperature can drop during reaction Examples: Photosynthesis, baking, chemical "ice" packs |
|
|
Exothermic Reaction |
when compounds release energy to form their products; produce light or heat during the reaction |
|
|
Reactants |
chemicals before the chemical reaction |
|
|
Products |
chemicals that result from a chemical reaction |
|
|
Chemical Formula |
describes how many atoms and what types are involved in a chemical bond |
|
|
Why Bonds Form |
atoms fill their valence shells during a bond (to get stable) |
|
|
Why Chemical Reactions Occur |
some type of energy breaks chemical bonds |
|
|
Coefficient |
number that describes how many moles (molecules) of a compound/molecule are involved in a reaction |
|
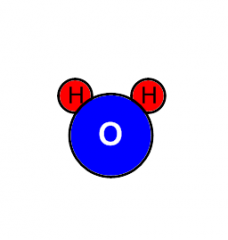
Chemical Formula |
H2O |
|

|
2 H2O |

