![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
82 Cards in this Set
- Front
- Back
|
How can you make an acid/alkali less hazardous?
|
You can dilute it.
|
|
|
Metal+Oxygen=
|
Metal Oxide
|
|
|
Metal + Water=
|
Metal Hydroxide
|
|
|
What happens to the contents in a oxidation or combustion reaction?
|
The contents increase because they have gained oxygen.
|
|
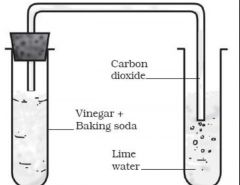
What is the test for carbon dioxide?
|
The test for carbon dioxide is when limewater turns cloudy
|
|

What is the test for hydrogen?
|
A lighted wooden splint in a test tube and if there is a squeaky pop-hydrogen was produced.
|
|
|
What gas is present in all acids?
|
Hydrogen
|
|
|
Why do bases react with acids?
|
Bases react with acids to neutralise them.
|
|
|
When heat is added to a metal carbonate, it decomposes to what?
|
Metal Oxide and Carbon Dioxide
|
|
|
How can you protect a metal from corrosion?
|
Metals can be painted to be protected from corrosion
|
|
|
Metal + Acids =
|
Salt+Hydrogen
|
|
|
Metal Oxides+Acid=
|
Salt+Water
|
|
|
Metal Hydroxides + Acid =
|
Salt+Water
|
|
|
Metal Carbonates + Acid =
|
Salt+Water+Carbon Dioxide
|
|
|
What are bases?
|
An substance capable of reacting with an acid to form salt and water.
|
|
|
What is an alkali?
|
A base that is dissolved in water.
|
|
|
What are hydroxides?
|
Hydroxides are bases that are dissolved in water.
|
|
|
Are all alkalis bases?
|
Yes
|
|
|
Why aren't all bases alkalis?
|
Because not all bases can be dissolved in water.
|
|
|
What is a displacement reaction?
|
When a more reactive metal replaces a less reactive metal from a compound.
|
|
|
What is a neutralisation reaction?
|
It is the chemical reaction between an acid and a base
|
|
|
What is combustion and its word equation?
|
The process of burning. Metal + Oxygen = Metal Oxide
|
|
|
What is oxidation?
|
When a substance is combined with oxygen
|
|
|
What is thermal decomposition?
|
When heat is added the compound breaks down
|
|
|
What is precipitation?
|
When two soluble solutions form an insoluble solid.
|
|
|
Name the most reactive metals:
|
Potassium,Sodium,Calcium,Magnesium,Aluminium
|
|
|
Name the medium reactive metals:
|
Zinc,Iron,Lead and Copper
|
|
|
Name the unreactive metals:
|
Sliver and Gold
|
|
|
How are reactive metals extracted and why?
|
By electrolysis which means electricity is used to break. It is the only method that can be strong enough to break the metal-oxygen bond.
|
|
|
How are medium reactive metals extracted?
|
They are extracted by carbon reduction and electrolysis. But carbon reduction is a cheaper method.
|
|
|
How are unreactive metals extracted?
|
They are extracted by mining.
|
|

What is a physical change?
|
A change of state/appearance but its a reversible change.
|
|

What is a chemical change?
|
A change of appearance its a irreversible change.
|
|
|
What is the main chemical in rocks?
|
Calcium Carbonate (Limestone)
|
|
|
Why were more reactive metals discovered later?
|
They were discovered later because they were hard to find in their combined form and couldn't have been extracted until the invention of Electrolysis in 1800's
|
|
|
Name these acids:
|
HCl=
HNO3= HSO4= HCO3= |
|
|
Name these acids:
|
HCl=
HNO3= HSO4= HCO3= |
|
|
Carbonate =
|
CO3
|
|
|
What makes up the fire triangle?
|

|
|
|
Do less reactive metals react with acids?
|
Yes less reactive metals only react with hot acids.
|
|
|
Do less reactive metals react with acids?
|
Yes less reactive metals only react with hot acids.
|
|
|
Describe what Tarnish is
|
Tarnish is the discolouration caused by the reaction between a metal and oxygen
|
|
|
How can you remove tarnish? What are the safety precautions?
|
You can remove tarnish by washing with a polish. However leaving moisture on its surface can cause corrosion.
|
|
|
How can you remove tarnish? What are the safety precautions?
|
You can remove tarnish by washing with a polish. However leaving moisture on its surface can cause corrosion.
|
|
|
How can iron objects be protected against rusting?
|
Iron objects can be prevented from rusting by preventing oxygen or water from reaching it.
|
|
|
Word Equation For Rusting:
|
Iron+Water+Oxygen = Hydrated Iron Oxide (rust)
|
|
|
Two solutions are mixed, a new substance is formed.
Is this a precipitation or decomposition reaction? |
Precipitation
|
|
|
A blue substance is heated. It turns black and a gas is given off. Is this a precipitation or decomposition reaction?
|
Decomposition
|
|
|
A blue substance is heated. It turns black and a gas is given off. Is this a precipitation or decomposition reaction?
|
Ndecomposition
|
|
|
A blue substance is heated. It turns black and a gas is given off. Is this a precipitation or decomposition reaction?
|
Decomposition
|
|
|
If there is less metal at the end of the reaction because it has been changed into a new substance. What word would you used to describe that?
|
Corrosion
|
|
|
Why do carbonates fizz when acids are added together?
|
They fizz because they give off a gas called carbon dioxide.
|
|
|
Why does it pop during the test for hydrogen gas?
|
Because the flame ignites the hydrogen
|
|
|
What happens if you test for carbon dioxide with a lighted splint?
|
It doesn't pop, the flame goes out.
|
|
|
Why do carbonates fizz when acids are added together?
|
They fizz because they give off a gas called carbon dioxide.
|
|
|
Why does it pop during the test for hydrogen gas?
|
Because the flame ignites the hydrogen
|
|
|
What happens if you test for carbon dioxide with a lighted splint?
|
It doesn't pop, the flame goes out.
|
|
|
Magnesium Hydroxide + Nitric Acid=
|
Magnesium Nitrate + Water
|
|
|
Why do carbonates fizz when acids are added together?
|
They fizz because they give off a gas called carbon dioxide.
|
|
|
Why does it pop during the test for hydrogen gas?
|
Because the flame ignites the hydrogen
|
|
|
What happens if you test for carbon dioxide with a lighted splint?
|
It doesn't pop, the flame goes out.
|
|
|
Magnesium Hydroxide + Nitric Acid=
|
Magnesium Nitrate + Water
|
|
|
What reactants would make Calcium Chloride + Water
|
Calcium Hydroxide+Hydrochloric Acid
|
|
|
Why do carbonates fizz when acids are added together?
|
They fizz because they give off a gas called carbon dioxide.
|
|
|
Why does it pop during the test for hydrogen gas?
|
Because the flame ignites the hydrogen
|
|
|
What happens if you test for carbon dioxide with a lighted splint?
|
It doesn't pop, the flame goes out.
|
|
|
Magnesium Hydroxide + Nitric Acid=
|
Magnesium Nitrate + Water
|
|
|
What reactants would make Calcium Chloride + Water
|
Calcium Hydroxide+Hydrochloric Acid
|
|
|
What do acids and alkalis react to form and in what reactions?
|
Acids and alkalis react to form salts in neutralisation reactions
|
|
|
How can you tell if a chemical reaction has taken place?
|
Colour, Temperature Change or a gas is given off indicated by bubbles or flames.
Something new is always formed |
|
|
How can you tell if a chemical reaction has taken place?
|
Colour, Temperature Change or a gas is given off indicated by bubbles or flames.
Something new is always formed |
|
|
What is Redox?
|
Reduction and Oxidation
|
|
|
How can you tell if a chemical reaction has taken place?
|
Colour, Temperature Change or a gas is given off indicated by bubbles or flames.
Something new is always formed |
|
|
What is Redox?
|
Reduction and Oxidation
|
|
|
Name the ingredients need for Combustion to take place:
|

|
|
|
Give some examples of bases:
|
Metal Oxide/Hydroxides/Carbonates
|
|
|
What is reduction?
|
The process or result of reducing or being reduced.
|
|
|
What is another name for displacement reactions?
|
Competition Reactions
|
|
|
Why would there be no reaction when copper and magnesium oxide react?
|
Because magnesium is more reactive than copper.
|
|
|
Zinc reacts with copper sulphate to become zinc sulphate and copper. What colour would the zinc used be now? If zinc is grey and copper is brown.
|
Browny grey
|
|
|
Answer and complete the following :
Calcium Carbonate + Nitric Acid = |
Calcium Nitrate
|
|
|
Answer and complete the following :
Calcium Carbonate + Nitric Acid = |
Calcium Nitrate+Water+Carbon Dioxide
|

