![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
What are the sp3 Carbon structure and bond angle?
|
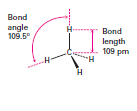
4 orbitals are
Tetrahedral |
|
|
What are the sp2 Carbon structure and bond angle?
|
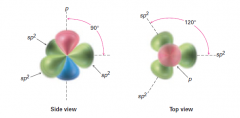
3 orbitals are Trigonal Planar
120 degrees |
|
|
What are the sp Carbon structure and bond angle?
|
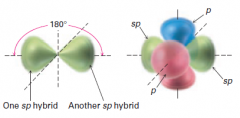
2 orbitals
Linear 180 degrees |
|
|
When is a COVALENT bond polar?
|
When the difference in EN is 0.5 or greater
|
|
|
Do alcohols have resonance structures?
|
No
|
|
|
Def of resonance structure
|
Different possible lewis structures for a molecule or ion
|
|
|
What is a Bronsted Acid?
|
Proton donor
Must have H+ to donate |
|
|
What is a Bronsted Base?
|
H+ acceptor
(have a lone pair) |
|
|
What Ka indicates a strong/weak acid?
|
strong 10^2 to 10^9
weak 10^-5 to 10^-15 |
|
|
What is a Lewis Acid?
Lewis base? |
A Lewis acid accepts an e- pair(no octet)
A Lewis base donates an e- |
|
|
Alkene
|

Double bond
|
|
|
Alkyne
|

Only C and H
Only sp3 Carbons CnH2n+2 |
|
|
Arene
|

|
|
|
Halide
|

|
|
|
Alcohol
|

|
|
|
Ether
|

|
|
|
Monophosphate
|

|
|
|
Diphosphate
|

|
|
|
Amine
|

|
|
|
Imine
(Schiff base) |

|
|
|
Nitrile
|

|
|
|
Thiol
|

|
|
|
Sulfide
|

|
|
|
Disulfide
|

|
|
|
Sulfoxide
|

|
|
|
Aldehyde
|

|
|
|
Ketone
|

|
|
|
Carboxylic Acid
|

|
|
|
Ester
|

|
|
|
Thioester
|

|
|
|
Amide
|

|
|
|
Acid chloride
|

|
|
|
Carboxylic acid anhydride
|

|
|
|
Rules: Naming Compounds
|
1. Find longest C chain and name it
2. Find branches and # them(lowest #s) 3. Name branches/substituents. Put in alphabetical order(iso is only prefix that counts) |
|
|
Rules: Drawing Lewis Structures
|
1. Count valence e- in molecule(add group #s)
2. Place least e- atom in center 3. Place other atoms around central atom 4. Put in bonds and LPs so each 2nd row atom has an octet. |

