![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
What is fluorescence?
|
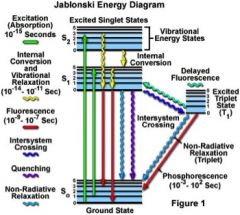
Occurs when a molecule relaxes to its ground state after exitation by emitting a photon
-Some organic compounds relax to the triplet state, then phosphoresce to return to ground, therefore not emitting a fluorescent photon. |
|
|
Quantum Yield
|
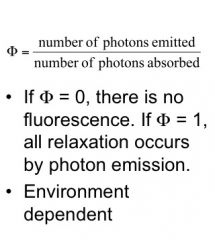
|
|
|
What types of molecules flouresce?
|
Conjugated pi-system (high e)
Rigid molecules that relax primarily through photon emission rather than non-radiatively |
|
|
Fluorescence brightness =
|
e x F
|
|
|
Some proterties of spectra
|
E=hv
v=c/wavelength Red=low E Violet=High E |
|
|
Quenching
|
Occurs when interactions with other molecules decrease flourescence (ie, another molecule absorbs emitted photon before it is seen)
|
|
|
Photobleaching
|
irreversible destruction of the fluorophore, often a result of reaction with singlet oxygen
|
|
|
Types of quenching
|
Collisional quenching: loss of excitation energy as heat rather than light
static quenching: formation of a stable complex with reduced flourescence (ie dimerization dyes) Photoinduced electron transfer (PET) Forster resonance energy transfer (FRET) |
|
|
Quenching by dimerization of dyes
|
static quenching: requires van der Waals contact
typically alters absorption spectra and lowers quantum yield |
|
|
Quenching by PET
|
photoinduced electron transfer
excitation of an electron into a higher energy state allows a donor to fill the lower energy state, and an acceptor at an intermediate energy state to accept the initial excited electron. Short range (<5 A) - VDW contact |
|
|
Quenching by FRET
|
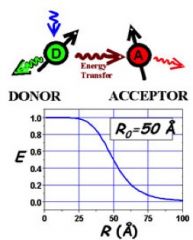
Through-space; 10-100Ǻ, typically 20-70 Ǻ.
|
|
|
common Fluorophor colors
|
Blue, green, red
|
|
|
First use of immunofluorescence
|
In 1950 Albert H. Coons covalently attached fluorescein isothiocyanate (FITC) to an antibody
|
|
|
Live cell imaging using fluorescence (not fluorescent proteins)
|
Microinjection of fluorescently-labeled protein into cells allows live cell imaging.
Ex: X-Rhodamine-labeled tubulin allows imaging of microtubule dynamics |
|
|
GFP
|
Green Fluorescent protein from Aequeria Victoria (crystal jelly) by Shimomura in the 1960s/1970s
First cloned by Prasher in 1992 first Expressed in E. coli: Chalfie, 1994 Can be expressed in cells |
|
|
Which AAs flouresce?
|
tryptophan
tyrosine phenylalanine |
|
|
Drawbacks of GFP
|
takes time to fold
needs proper environment |
|
|
Mutating GFP
|
scientists have made BFP, YFP, CFP
no RFP |
|
|
dsRED
|
-Gln-Tyr-Gly chromophore
-Tetrameric, oligomerizes -Intermediate green state -Maturation takes > 10 hours! |
|
|
dsRED mutations
|
QYG (DsRed, mRFP)
MYG (tomato) CYG (tangerine, banana) TYG (orange, strawberry, cherry) MWG (honeydew) |
|
|
Near IR flours
|
can be used in vivo (Image a whole mouse)
|
|
|
Dynamic Protein Localization techniques using fluors
|
FRAP
photocaged fluorophores Kaede: a molecular highlighter |
|
|
FRAP
|
Flourescence recovery after photobleaching
-used to study protein diffusion rates and compartmentalization |
|
|
photocaged fluorophores
|
"cage" is released upon activation with a particular wavelength, releasing fluorophor
|
|
|
Flourescent probes and sensors:
|
Environmental sensitivity
Modulation of quenching (PET, FRET) Latent fluorophores |
|
|
DNA binding dyes
|
have large increase in fluorescence upon binding DNA (intercalation, minor groove binding, ect.)
EtBr, Hoechst, sybr green |
|
|
Solvatochromic dyes
|
change colour according to the polarity of the liquid in which they are dissolved
|
|
|
Quenching as a probe:
|
Use reporter-quencher dual labeled probes
|
|
|
PET quenching by electron-rich molecules:
|
tryptophan and guanine
Must be in close proximity (VDW) <5 A |
|
|
PET-based sensors of zinc and NO
|
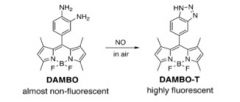
|
|
|
2 common PET-based assays
|
GTP binding using Bodipy-FL
Calcium sensors |
|
|
FRET uses
|
There are nucleotide and peptide FRET sensors
sensitive enough to see intramolecular interactions and even protein activation states FRET is commonly used in genetically encoded Ca sensors CFP-YFP are a common FRET pair due to little wavelength overlap |
|
|
Latent Flourophores
|
Commonly used Beta-lactam conjugated fluorophore, activated by Beta galactosidase cleavage
|

