![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Atom |
The smallest part of an element |
Atoms make up everything |
|
|
Atomic mass |
The mass of an atom in AMUs. The average mass of all natural isotopes |

The number at the bottom of the cell is the atomic mass |
|
|
Atomic mass unit |
One-twelfth the mass of Carbon-12 |

Atomic mass is measured in atomic mass units |
|
|
Atomic number |
The number of protons in an atom |
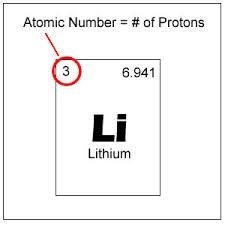
The atomic number identifies the element |
|
|
Atomic symbol |
The letter or letters that identify an atom |
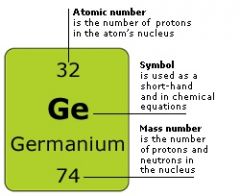
The symbol for germanium is Ge |
|
|
Electron |
An elementary particle with a negative charge |
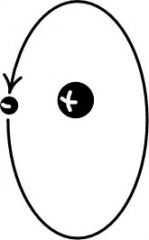
Electrons float around the nucleus |
|
|
Group |
Elements of a vertical column in the periodic table |
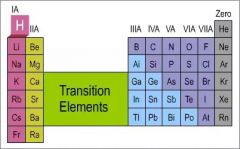
Hydrogen is in the first group |
|
|
Isotope |
Two or more atoms of the same element with different masses |
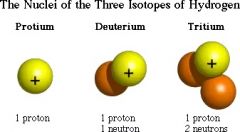
Hydrogen has three isotopes |
|
|
Mass number |
The total number of protons and neutrons in an atom |
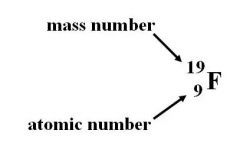
This fluorine has a mass number of 19 |
|
|
Metal |
An element that tends to lose electrons in chemical reactions |

Metals tend to be shiny and malleable |
|
|
Metalloid |
An element that has properties of metals and nonmetals |

Some metalloids look and act like metals |
|
|
Neutron |
A neutral subatomic particle |

Neutrons are called neutrons because they're neutral. |
|
|
Nonmetal |
An element that tends to gain electrons in chemical reactions |

Nonmetals are usually dull and brittle |
|
|
Nucleus |
The center of an atom. Has mass and a positive charge |
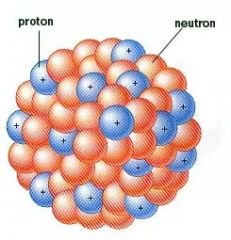
The nucleus is made up of protons and neutrons |
|
|
Period |
Elements of a horizontal row on the periodic table |
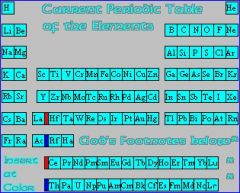
Helium is in the first period |
|
|
Proton |
A subatomic particle with a positive charge |
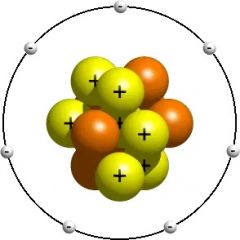
Protons make the nucleus positive |
|
|
Subatomic particle |
A particle that is smaller than an atom |

Protons, neutrons, and electrons are subatomic particles. |

