![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Salt Bridges
|
Positive and negative charges (strongest)
|
|
|
Dipole-dipole interations
|
Polar Bonds (2nd weakest)
|
|
|
Hydrogen Bonds
|
Look for H atom bonded to N,O, or F. (Only need O, N, F, or partial negative charge to H bond with water)
|
|
|
Ion-Dipole Bond
|
Polar bond and + or - charge (2nd Strongest)
|
|
|
London Forces
|
All molecules (weakest)
|
|
|
IUPAC
|
International Union of Pure and Applied Chemistry
|
|
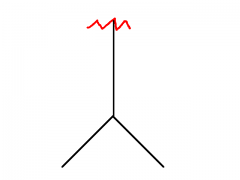
|
Isopropyl
|
|

|
Iso-butyl
|
|
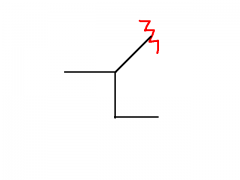
|
Sec-butyl
|
|
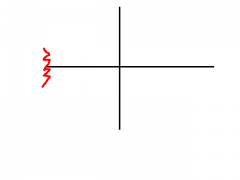
|
Tert-butyl
|
|
|
Constitutional isomers
|
Same formula but different name
|
|
|
Conformations
|
Same formula and name but different 3-D structure
|
|
|
Stereoisomers
|
Same formula and connections but different 3-D shape
|
|
|
Geometric isomers
|
Stereoisomers due to restricted bond rotation (cis and trans)
|
|
|
Saturated
|
hydrocarbons that only contain single bonds
|
|
|
Unsaturated
|
hydrocarbons with double or triple bonds
|
|
|
Aromatic compounds
|
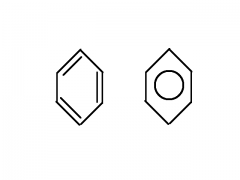
Benzene rings
|
|
|
Numbering benzene
|

|
|
|
Functional groups
|
An atom, group of atoms, or bond that gives a molecule a characteristic set of chemical properties
|

