![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
84 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
# Of Elements |
113 |
|
|
|
# Of Synthetic Elements |
23 |
|
|
|
What is chemistry the study of? |
Study of Matter & Changes it undergoes |
|
|
|
Matter - Definition |
Anything w/ mass that takes up space |
|
|
|
Elements - Definition |
Made of 1 thing from periodic table |
|
|
|
Compounds - Definition |
Combination of 2+ Elements |
|
|
|
What is a mixture made up of? |
Mix of both elements and compounds |
|
|
|
Atomic Theory |
The belief all matter is made up of atoms/combinations of atoms |
|
|
|
What are atoms? |
-Smallest particle of element -Neutral Charge |
|
|
|
True or False - Atoms are Electrically Neutral |
True |
|
|
|
Nucleus - Definition |
-Center of atom -Positivley charged |
|
|
|
What makes up most of an atoms mass? |
The nucleus |
|
|
|
Electrons |
Orbits nucleus |
|
|
|
Atomic Mass Formula |
AM = p+ + n |
|
|
|
WHIMIS Abbreviation |
Workplace Hazardous Material Information System |
|
|
|
What is WHIMIS? |
Symbols for dangerous substances you can't get over the counter |
|
|

|
Dangerously Reactive Materials |
|
|
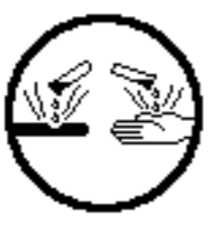
|
Corrosive Material |
|
|

|
Biohazardous Infectious Material |
|
|

|
Poisonous and Infectious Material (Insta Death/Harm) |
|
|
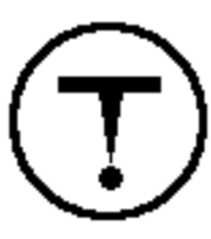
|
Poisonous and Infectious Material (Causing other toxic effects) |
|
|
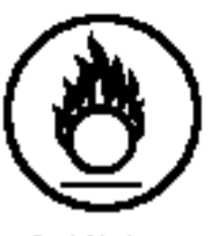
|
Oxidizing Material (easily flammable in oxygen) |
|
|
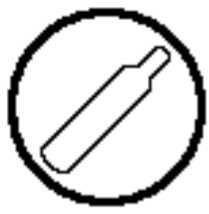
|
Compressed Gas |
|
|

|
Flammable & Combustible Material |
|
|
|
Hazardous Household Product Symbols |
Self explanatory |
|
|

|
Poison |
|
|

|
Corrosive |
|
|
|
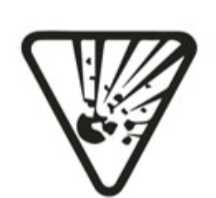
Explosive |
|
|

|
Flammable |
|
|
|
What info is included with WHIMIS products? |
-First aid/safety info -Storage info -Reccomended PPE -Cleaning/Handling info |
|
|
|
What does MSDS Stand for? |
Material Safety Data Sheet |
|
|
|
Mixtures - Mechanical |
Individual parts are easily seen and separated |
|
|
|
Mixtures - Solution |
Combined, looks like one uniform substance |
|
|
|
Solute |
-Material being dissolved -Smaller Quantities |
|
|
|
Solvent |
-Material doing the dissolving -Larger Quantities |
|
|
|
Pure Substances Type - Element |
Made of one single element |
|
|
|
What is a compound? |
-Made of 2 or more elements -Pure Substance |
|
|
|
Pure Substances Types |
Elements & Compounds |
|
|
|
Mixtures Types |
Mechanical & Solution |
|
|
|
Homogeneous |
Visually distinguishable components |
|
|
|
Heterogenous |
Appear uniform |
|
|
|
Dalton's Model |
|
|
|
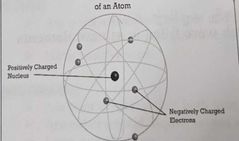
Planetary Model |
|
|
|
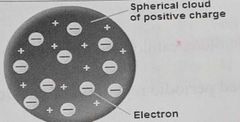
Thomson's Plum Pudding Model |
|
|

|
Electron Cloud Model |
|
|
|
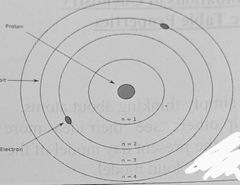
The Bohr Model |
|
|
|
Thomson's Plum Pudding Model |
-1st scientist to find out about + and - charges -Sphere of positive charge w/ negative charges embedded inside |
|
|
|
Dalton's Model Info |
AKA Hard sphere/billiard ball model -Solid Spheres w/ no internal structure |
|
|
|
Planetary Model of an Atom |
AKA Rutherford/nuclear Model -Small nucleus contains most of mass w/ negative electrons randomly orbiting -Mostly empty space |
|
|
|
The Bohr Model |
-Elecyrons orbit at specific fixed distances |
|
|
|
Electron Cloud Model |
AKA Quantum Mechanical Model -Most Accurate -Location predicted with probability and statistics
|
|
|
|
What is a group/family? |
Vertical Rows on periodic table -Similar Traits |
numbered in the booklet |
|
|
What are periods? |
Horizontal rows |
|
|
|
Alkali Metal - Location |
Group 1 |
|
|
|
Alkali Metal - Definition |
Highly reactive metal element |
|
|
|
Alkali Earth Metals - Location |
Group 2 |
|
|
|
Alkali Earth Metal - Definition |
Shiny, white, semireactive elements |
|
|
|
Halogens - Location |
Group 17 |
|
|
|
Halogens - Definition |
Reactive Non-Metals |
|
|
|
Nobel Glasses - Location |
Group 18 |
|
|
|
Most Reactive Metal |
Fr - Francium |
|
|
|
Most Reactive Non-Metal |
F - Fluorine |
|
|
|
Metals Characteristics |
-Good heat and electricity conductors -Solid at room temp -Positive Ions |
|
|
|
Non-Metals Characteristics |
-Poor conductors -Can be Solid, liquid or gas at room temp -Negative Ions |
|
|
|
Metalloids Characteristics |
-Have properties of both metals and non metals -May or may not form ions |
|
|
|
Valence Electrons - Definition |
# of electrons in outermost level |
|
|
|
Valence Electrons & Groups |
#of electrons in outermost level = group number ex) Element in group 2=2 Ve- |
|
|
|
Energy levels & Periods |
# of energy levels occupied by electrons = period # |
|
|
|
What are ions? |
atoms w/ a charge due to loss/gain if electrons -either always results in full valence level |
|
|
|
Loss of electrons = ? |
Positive Ion |
|
|
|
Gain of electrons = ? |
Negative Ion |
|
|
|
What are Cations? |
Positive Ions |
|
|
|
Metal Ions - Name change |
Name doesn't change, only becomes [Element] Ion ex) Iron Ion |
|
|
|
What forms negative ions by gaining electrons on their outside level? |
non metal Ions |
|
|
|
Anions |
Negative Ions |
|
|
|
Non Metal Ions - Name Change |
"Ide" is attached to ending ex) Fluorine = Fluoride |
|
|
|
Why are ions gained or lost? |
To become more stable |
|
|
|
Who created the 1st periodic table? |
Dimitri Mendeleev |
|
|
|
What are transition metals? |
The metals between alkaline earth metals and metalloids |
|
|
|
What determines the size/volume of an atom |
The Electrons |
|
|
|
Are negative ions gained or lost? |
Gained |
|
|
|
Are positive Ions gained or lost? |
Lost |
|
|
|
What loses their outside levels? |
Metals |
|
|
|
What fills their outside levels? |
Non Metals |
|

