![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
93 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Color is a _____ property. |
Intensive |
|
|
|
Mass, volume, and length are all ________ properties. |
Extensive |
|
|
|
Density and melting/boiling points are _______ properties. |
Intensive |
|
|
|
An intensive property is __________ of the amount of substance present. |
Independent. |
|
|
|
Extensive properties are _________ on the same amount of substance present. |
Dependent. |
|
|
|
What is the SI unit for length and it’s symbol? |
Meter : m |
|
|
|
What is the SI unit for mass and it’s symbol? |
Kilogram : kg |
|
|
|
What is the SI unit for mass and it’s symbol? |
Kilogram : kg |
|
|
|
What is the SI unit for Time and it’s symbol? |
Second : s |
|
|
|
What is the SI unit for temperature? And what is its symbol? |
Kelvin : K |
|
|
|
What is the formula to convert Celsius to Fahrenheit? |
F = (9/5)(C) + 32 |
|
|
|
What is the formula to convert Celsius to Fahrenheit? |
F = (9/5)(C) + 32 |
|
|
|
What is the formula to convert Fahrenheit to Celsius? |
C = 5/9 (F - 32) |
|
|
|
To convert Celsius to Kelvin you ADD ______. |
273.15 |
|
|
|
To convert Kelvin to Celsius you subtract ____. |
273.15. |
|
|
|
What is the formula for density? |
D = mass/volume |
|
|
|
How many sig figs? : 0.0234. |
3 sig figs. |
|
|
|
How many sig figs? : 2.300x10^-2. |
4 sig figs. |
|
|
|
How many sig figs? : 20000. |
At least one. |
|
|
|
How many sig figs? : 0.0003. |
1 sig fig. |
|
|
|
____ is how close to the known value you are. |
Accuracy. |
|
|
|
____ Is how close together your data points are. |
Precision |
|
|
|
What is the formula for Kelvin? |
K = C + 273.15 |
|
|
|
Which scientist discovered the electron? |
JJ Thomson. |
|
|
|
Who discovered the charge of the electron using oil drops? |
Millikan. |
|
|
|
Which scientist suggested that atoms resemble plum pudding? |
Thomson. |
|
|
|
Which scientist proposed that atoms resembled the planet Saturn? |
Nagaoka. |
|
|
|
________ & ______ are the scientists who fired alpha particles at a piece of gold foil and detected where those particles went. |
Geiger & Rutherford |
|
|
|
The majority of the volume of an atom is made up of what? |
Empty space. |
|
|
|
How do you determine the atomic number of an element? |
It is the number of protons. |
|
|
|
Does the atomic number ever change? |
No, the atomic number is the number of protons, and it never changes. |
|
|
|
How do you determine the mass number of an element? |
It is the number of protons and neutrons. |
|
|
|
_________ are atoms which differ in mass from assigned mass of an element but have the same chemical properties of the element. |
Isotopes. |
|
|
|
Why is an isotope different? |
They differ in the number of neutrons which results in different masses. |
|
|
|
Atomic ______ = Number of protons. |
Number |
|
|
|
Atomic ______ = Number of protons and neutrons. |
Mass |
|
|
|
Atomic ______ = Number of protons minus electrons. |
Charge. |
|
|
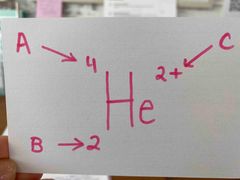
What is A? |
The mass #. |
|
|
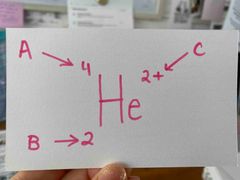
What is B? |
The atomic #. |
|
|
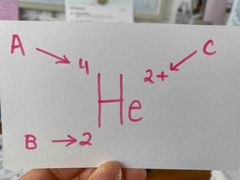
What is C? |
The charge. |
|
|
|
What is the formula for calculating the average mass of an element? |
Average mass = E (fractional abundance x isotopic mass) |
|
|
|
1 hour = _____ seconds. |
3600. |
|
|
|
1 mm = ____ cm. |
0.1 |
|
|
|
1KL = ______ L |
1000 |
|
|
|
1 m = _______ inches. |
39.37. |
|
|
|
1 inch = ______ cm. |
2.54. |
|
|
|
1 m = ____ ft. |
3.28 |
|
|
|
1 ft = _____ m. |
0.305. |
|
|
|
1 km = ______ m. |
1000 |
|
|
|
1 kcal = ______ calories |
1000 |
|
|
|
1 kg/L = _____ kg/mL |
0.001 |
|
|
|
1 g = _____ mg |
1000 |
|
|
|
1 cm = ____ mm. |
10. |
|
|
|
1 mile = _____ km |
1.609 |
|
|
|
1 g = ____ mg |
1000 |
|
|
|
1 m = _____ yards |
1.094 |
|
|
|
True or false: 1 cm^3 = 1 mL |
True |
|
|
|
1 L = _____ mL |
1000 |
|
|
|
What is the prefix & symbol for: 1,000,000 & 10^6. |
Mega : M |
|
|
|
What is the prefix & symbol for: 1000 & 10^3. |
Kilo : k |
|
|
|
What is the prefix & symbol for: 0.1 & 10^-1. |
Deci : d |
|
|
|
What is the prefix & symbol for: 0.01 & 10^-2. |
Centi : c |
|
|
|
What is the prefix & symbol for: 0.001 & 10^-3. |
Milli : m |
|
|
|
What is the prefix for: 0.000001 & 10^-6. |
Micro |
|
|
|
The _______ formula shows exactly how many atoms there are. |
Molecular. |
|
|
|
The ______ formula is the lowest while number ratio of atoms. |
Empirical. |
|
|

Is this formula for glucose empirical or molecular? |
Empirical. |
|
|
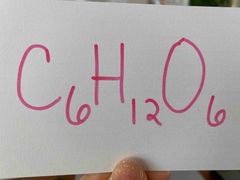
Is this formula for glucose empirical or molecular? |
Molecular. |
|
|
|
______ isomers have the same formula but different structures (and therefore different chemical properties). |
Structural isomers. |
|
|
|
______ isomers have the same formula but different structures (and therefore different chemical properties). |
Structural isomers. |
|
|
|
_____ isomers only differ in the relative orientations of the atoms in space. |
Spatial isomers. |
|
|
|
Who is the scientist credited with creating the first periodic table of elements? |
Mendeleev. |
|
|
|
There are ____ horizontal rows (“periods”) and ____ vertical columns (“groups”) in the periodic table. |
7 ; 18 |
|
|
|
______ is a suffix used for a lower charge cations, _____ is a suffix used for higher charge cations. |
-ous ; -ic |
|
|
|
_____ are polyatomic and anions that contain oxygen. |
Oxyanions. |
|
|
|
When there are two oxyanions involving the same element, the one with fewer oxygens ends in ______ and one with more oxygens ends in ______. |
-ite ; -ate |
|
|
|
T/F: Sulfate has more oxygens that sulfite. |
True!!! Oxyanions involving the same element with more oxygens end in -ate. Those with less oxygens end in -ite. |
Funny: I ate more food than Ite. |
|
|
Oxyanion nomenclature- halogens: The one with the second fewest oxygens ends in _____. |
-ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the second fewest oxygens ends in _____. |
-ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the second most oxygens ends in _____. |
-ate |
|
|
|
Oxyanion nomenclature- halogens: The one with the second fewest oxygens ends in _____. |
-ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the second most oxygens ends in _____. |
-ate |
|
|
|
Oxyanion nomenclature- halogens: The one with the fewest oxygens has the prefix _____ and ends in _____. |
hypo- ; -ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the second fewest oxygens ends in _____. |
-ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the second most oxygens ends in _____. |
-ate |
|
|
|
Oxyanion nomenclature- halogens: The one with the fewest oxygens has the prefix _____ and ends in _____. |
hypo- ; -ite |
|
|
|
Oxyanion nomenclature- halogens: The one with the most oxygens has the prefix _____ and ends in ______. |
per- ; -ate |
|
|
|
Ionic compound formulas must be _______ neutral and are always written as ________ formulas. |
Electrically ; empirical |
|
|
|
The reaction between a metal and a nonmetal produces a ________ compound. |
Ionic. |
|
|
|
The attraction between positive and negative ions is called the ____________ which forms an ionic bond between the atoms. |
Electrostatic force |
|
|
|
One millimeter is equal to how many meters? A) 10^3 B) 10^-3 C) 10^2 D) 10^-6 |
B) 10^3 |
|
|
|
1 gal = ____ qt |
4 |
|
|
|
1 qt = _____ L |
.94635 |
|

