![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
Atom |
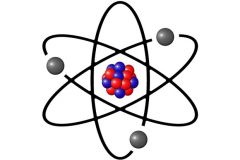
Basic units of matter |
|
|
Atomic mass |
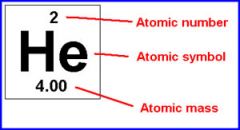
Average weighted mass of all isotopes in a naturally occurring sample of that element. |
|
|
Atomic mass unit |
1/12 the mass of carbon-12 and used to express the mass of atomic and subatomic particles. |
|
|
Atomic number |
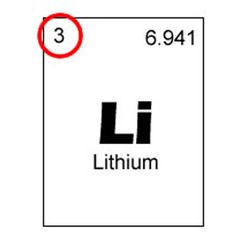
The number of protons in the nucleus of an atom. Also used to describe the elements place on the periodic table. |
|
|
Calibration curve (lab) |
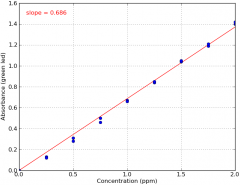
A graph |
|
|
Concentration (lab) |

Measure of the quality of solute dissolved in a solution at a given temperature.
|
|
|
Conversion factor |
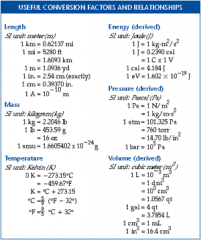
Comparing U.S. units to metric units. |
|
|
Density (lab) |

Ratio of mass per unit of volume. Does not depend on the amount of substance. (Intensive) |
|
|
Electron |
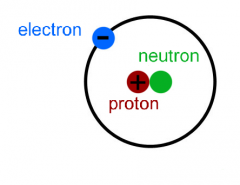
A negatively charged particle that floats around outside the nucleus of an atom in the electron cloud. |
|
|
Exact number |
When the exact number of the unit being counted is calculated, this is the result. |
|
|
Inexact number |

Numbers whose value have some uncertainty and aren't precise. |
|
|
Ion |
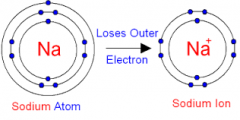
positively or negatively charged particles by gaining or loosing electrons. |
|
|
Isotope |

Contain equal numbers of protons but different numbers of neutrons in the nucleus. |
|
|
Mass (lab) |
Measure of an objects resistance to acceleration |
|
|
Mass number |
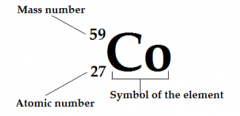
Protons + neutrons in the nucleus of the atom |
|
|
Neutron |
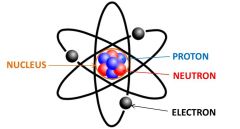
Neutrally charged particle located in the nucleus. |
|
|
Nucleus |
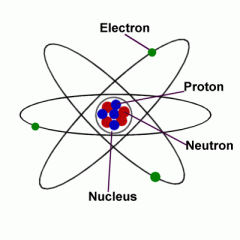
Core of atom contain protons and neutrons. |
|
|
Percent error (lab) |

Used to determine the precision of your calculations. |
|
|
Proton |
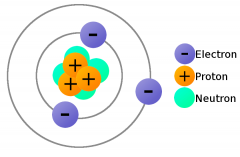
Positively charged atom located in the nucleus of an atom |
|
|
Scientific notation |
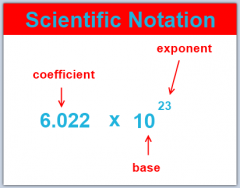
Displaying numbers in terms of a decimal number between 1 and 10 multiplied by 10 to a power. |
|
|
Significant digits |

Presicion based on most imprecise measurement |
|
|
Solution (lab) |
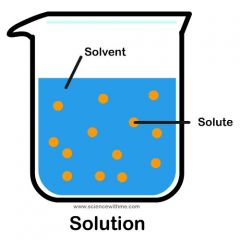
Homogenous mixture comprised of 2 or more substances. |
|
|
Solvent (lab) |
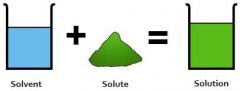
Substance doing the dissolving |
|
|
Subatomic particle |
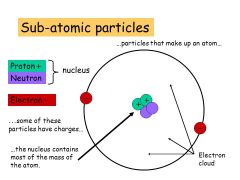
Particle smaller then an atom |
|
|
Volume (lab) |

The amount of space that a substance or object occupys.
|

