![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
Nitrate |
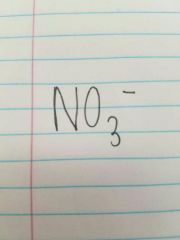
|
|
|
Bicarbonate |
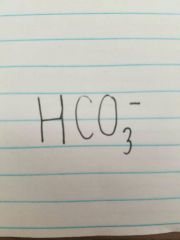
|
|
|
Chlorate |
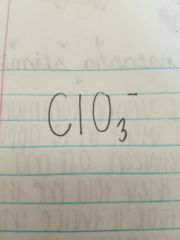
|
|
|
Hypochlorite |
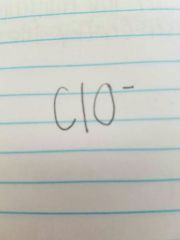
|
|
|
Cyanide |

|
|
|
Hydroxide |

|
|
|
Acetate |
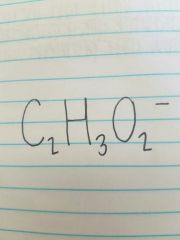
|
|
|
Sulfate |

|
|
|
Carbonate |
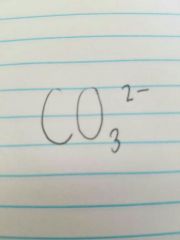
|
|
|
Peroxide |
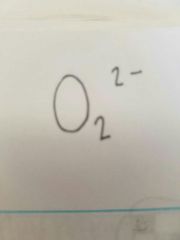
|
|
|
Phosphate |
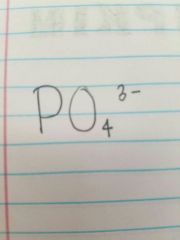
|
|
|
Ammonium |
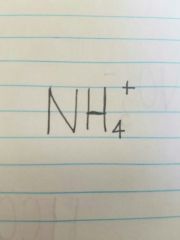
(only polyatomic ion) |
|
|
Valency |
# of electrons lost, gained, or shared during chemical reactions |
|
|
Chemical Reaction |
conversion of 1 or more substances to 1 or more NEW substances |
|
|
Chemical Equation |
represents a chemical reaction using symbols |
|
|
Aqueous means... |
soluble in H20 |
|
|
Arrows mean... |
"produces" or "yields" |
|
|
Chemical equations must be balances in accordance with... |
the law of conservation of mass |
|
|
To balanace equations... |
ensure that number of atoms of each element on the reactant side equals number of atoms of each element on the product side |
|
|
Acids... |
when dissolved in water provide hydrogen ions (H+) |
|
|
Bases... |
produce hydroxide ions in water (react in aqueous solutions) |
|
|
Nitrite |
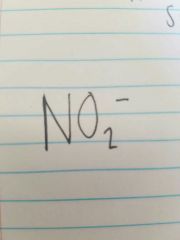
|
|
|
Nitride |
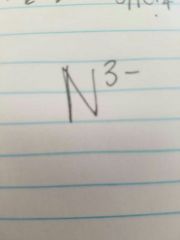
|
|
|
Sulfite |
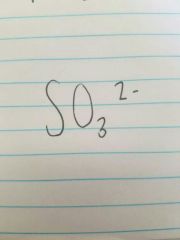
|
|
|
Sulfide |
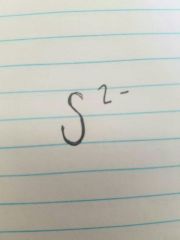
|
|
|
Phosphite |
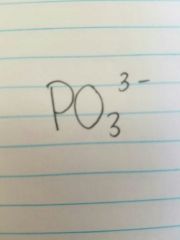
|
|
|
Phosphide |
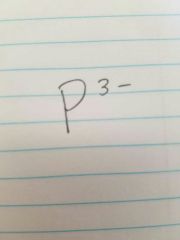
|
|
|
If acids are binary... |
name starts with "hydro" followed by modificatuon of non-metal name to "ic" |
|
|
If acids are not binary... |
modify name of the 1st element in the polyatomic ion to "ic" if "ate" and "ous" if "ite" |
|
|
Group 1 |
alkaline metals (+1) |
|
|
Group 2 |
alkaline-earth metals (+2) |
|
|
Group 3 |
(+3) |
|
|
Group 4 |
no charge |
|
|
Group 5 |
(-3) |
|
|
Group 6 |
(-2) |
|
|
Group 7 |
Hallogens (-1) |
|
|
Group 8 |
Noble gases, relativley unreactive |
|
|
Xenon (Xe) is useful for... |
MRI's |
|
|
Diatomic Molecules... |
Br, H, Cl, F, O, N, I |
|
|
Most Abundant Elements |
H, O, N, C |
|
|
In humans, heme group consists of... |
Iron (Fe) |
|
|
In plants, chlorophyll consists of... |
Magnesium (Mg) |
|
|
Ionic compounds are... |
GENERALLY CONSIST OF METAL CATION AND NONMETAL ANION (ex. NaCl) Crystalline solid, hard, brittle, high mp., bp., density, and electro |
|
|
Molecular compounds are... |
ONLY NON-METALS (ex. H2O) gas, liquid, solid, soft, low mp., bp., density, and electro |
|
|
Anion... |
negativley charged, found by gaining electrons |
|
|
Cations... |
positivley charged, formed by losing electrons |
|
|
Electronic Configuration... |
arrangement of electrons on shells around the nucleus of an atom |
|
|
Relative Atomic Mass |
% abundance divided by 100 × mass (amu) PLUS etc. (continue adding isotopes) |

