![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Heat energy change=mass*specific heat*change in temperature. |
Q=M*C*change in T You can solve for any variable depending on what the question is asking. |
|
|
Specific Heat |
Specific heat is the amount of heat energy needed to raise temperature of one gram of substance in a specific physical state by one Celsius. |
|
|
1 cal = ? |
4.184 Joules |
|
|
Avogrado's Law |
N1/V1=N2/V2 |
|
|
Combined Gas Law |
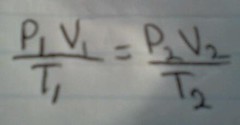
|
|
|
Acid base Pair |
The acid has one more acidic H atom and one fewer negative change than the base The base always has one fewer acidic H atom and one more negative charge than the acid. When an acid donates a proton the species that remains is called the conjugate base. When a base accepts a proton, the specie it becomes the conjugate acid. |
|
|
Strongest Conjugate Acid |
Nitric and sulfuric acids are strong acids |
|
|
What did Arrhenius say about acids and bases? |
Acids produce hydrogen ions in aqueous solutions, whereas bases produce hydroxide ions. |
|
|
IDEAL GAS LAW |
PV=NRT
N=MOLESR=.0821 |
|
|
1 ATM= |
760 TORR 14.68 PSI |

