![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
151 Cards in this Set
- Front
- Back
|
reaction of acid and bases with metal |
mg (s) + hcl (aq) -> MgCl2 (aq) +H2 (g) METAL + ACID -> salt (ionic compound but cation is not H+ or anion is not OH=) + hydrogen gas |
|
|
reaction of acid and bases with carbonate and bicarbonate |
H2SO4 +Na2CO3 -> Na2SO4 + H2CO3 (H2O + CO2) acid + carbonate -> salt + water + co2 |
|
|
buffers are all about |
because acids react and form h2co3 (carbonic acid) |
|
|
reaction of acid and bases with each other (acid +base->neutralization) |
hcl +naOH -> NaCl +H2O acid + base -> salt + water |
|
|
optimum ph for digestion |
2 |
|
|
Acids react with metals to produce |
salt and hydrogen gas. |
|
|
Acids react with bases to produce |
a salt of the metal and water. |
|
|
Acids react with bicarbonate and carbonate ions to produce |
carbon dioxide gas, salt, and water |
|
|
carbonate and bicarbonate are |
bases in body |
|
|
A salt is an ionic compound that |
does not have H+ as the cation or OH− as the anion. |
|
|
In a neutralization reaction,an acid reacts with a |
base to produce salt and water. the salt formed is the anion from the acid and the cation from the base. |
|
|
In neutralization reactions, |
one H+ always reacts with one OH−. |
|
|
Mg(OH)2 (s) + HBr(aq) -> |
salt + H2O(l) - Mg(OH)2 (s) + 2HBr(aq) salt + H2O(l) - Mg(OH)2 (s) + 2HBr(aq) salt + 2H2O(l) - Mg(OH)2 (s) + 2HBr(aq) MgBr2(aq) + H2O(l) |
|
|
A soft drink contains |
phosphoric acid (H3PO4) and carbonic acid (H2CO3). |
|
|
Arrhenius acids |
produce hydrogen ions (H+) when they dissolve in water. - are also electrolytes, because they produce H+ in water. have a sour taste. turn blue litmus red. corrode some metals. |
|
|
Acids with a hydrogen ion (H+) and a nonmetal (or CN−) ion are named |
with the prefix hydro and end with ic acid. HCl(aq) hydrochloric acid |
|
|
Acids with a hydrogen ion (H+) and a polyatomic ion are named |
by changing the end of the name of the polyatomic ion from -ate to ic acid - ite to ous acid |
|
|
HCl |
hydrochloric acid anion = Cl- name of anion = Chloride |
|
|
HBr |
hydrobromic acid anion = Br- name of anion = bromide |
|
|
HI |
hydroiodic acid
anion = I- name of anion = iodide |
|
|
HCN |
hydrocyanic acid anion = CN- name of anion = Cyanide |
|
|
HNO3 |
Nitric acid anion = NO3- name of anion = nitrate |
|
|
HNO2 |
Nitrous acid anion = NO2- name of anion = nitrite |
|
|
H2SO4 |
sulfuric acid anion = SO4 ^2- name of anion = sulfate |
|
|
H2SO3 |
Sulfurous acid anion = SO3^2- name of anion = sulfite |
|
|
H2CO3 |
carbonic acid anion = CO3^2- name of anion = carbonate |
|
|
HC2H3O2 |
acetic acid anion = C2H3O2- name of anion = acetate |
|
|
H3PO4 |
phosphoric acid anion = PO4^3- name of anion = phosphate |
|
|
H3PO3 |
phosphorous acid anion = PO3^3- name of anion = phosphite |
|
|
HClO3 |
chloric acid anion = ClO3- name of anion = chlorate |
|
|
HClO2 |
chlorous acid anion = ClO2- name of anion = chlorite |
|
|
The name of an acid with a hydrogen ion (H+) and a nonmetal uses |
the prefix hydro and ends with ic acid. - hydrobromic acid HBR |
|
|
An acid with a hydrogen ion (H+) and a polyatomic ion ending in ate is called an. |
ic acid - carbonic acid - H2CO3 |
|
|
An acid with a hydrogen ion (H+) and a polyatomic ion ending in ite is called an |
ous acid - bromous acid - HBrO2 |
|
|
Arrhenius bases |
produce hydroxide ions (OH−) in water. taste bitter or chalky. are also electrolytes, because they produce hydroxide ions (OH−) in water. feel soapy and slippery. turn litmus indicator paper blue and phenolphthalein indicator pink. |
|
|
NaOH |
soddium hydroxide |
|
|
KOH |
potassium hydroxide |
|
|
Ba(OH)2 |
barium hydroxide |
|
|
Al(OH)3 |
aluminum hydroxide |
|
|
acids |
- produce H+ - has electrolytes - tastes sour - feel may sting - litmus red - phenolphthalein colorless - neutralizes bases |
|
|
bases |
- produce OH- - has electrolytes - tastes bitter, chalky - feel soapy, slippery - litmus blue - phenolphthalein pink - neutralizes acids |
|
|
HNO2 |
nitrous acid |
|
|
Ca(OH)2 |
calcium hydroxide |
|
|
H2SO4 |
sulfuric acid |
|
|
HIO3 |
iodic acid |
|
|
NaOH |
sodium hydroxide |
|
|
bronsted lowry thoery |
acid donates h+ base accepts h+ |
|
|
_______donates h+ - bronsted lowry theory |
acid |
|
|
_______ accepts h+ - bronsted lowry theory |
base |
|
|
In any acid–base reaction, there are two conjugate acid–base pairs. |
Each pair is related by the loss and gain of H+. One pair occurs in the forward direction. One pair occurs in the reverse direction. |
|
|
Substances that can act as both acids and bases are |
amphoteric or amphiprotic. |
|
|
Water donates H+ when |
it reacts with a stronger base. |
|
|
Water accepts H+ when |
it reacts with a stronger acid. |
|
|
________only partially dissociate in water. |
Weak acids |
|
|
__________ is the only halogen that forms a weak acid. |
Hydrofluoric acid, HF, |
|
|
this complely ionizes (100%) in aqueous solutions - HCl |
strong acid |
|
|
this dissociates only slightly in water to form few ions in aqueous solutions - H2CO3 |
weak acid |
|
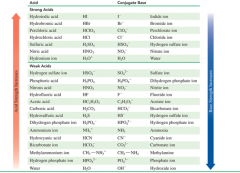
|
KNOW |
|
|
- have two H+ which dissociate one at a time - carbonic acid |
diprotic acids |
|
|
Strong bases as strong electrolytesare formed from |
metals of Groups 1A (1) and 2A (2). include LiOH, NaOH, KOH, Ba(OH)2, Sr(OH)2, and Ca(OH)2. dissociate completely in water. KOH(s) K+(aq) + OH−(aq) are found in household products used to remove grease and unclog drains. |
|
|
Weak bases are weak electrolytesthat are |
poor acceptors of H+ ions. produce very few ions in solution. include ammonia. |
|
|
Strong Bases |
Lithium hydroxide LiOH Sodium hydroxide NaOH Potassium hydroxide KOH Rubidium hydroxide RbOH Cesium hydroxide CsOH Calcium hydroxide Ca(OH) 2* Strontium hydroxide Sr(OH) 2* Barium hydroxide Ba(OH)2* *Low solubility, but they dissociate completely |
|
|
Weak Bases |
Window cleaner, ammonia, NH3 Bleach, NaOCl Laundry detergent, Na2CO3, Na3PO4 Toothpaste and baking soda, NaHC3 Baking powder, scouring powder, Na2CO3 Lime for lawns and agriculture, CaCO3 Laxatives, antacids, Mg(OH) 2, Al(OH)3 Strong Bases = Drain cleaner, oven cleaner, NaOH |
|
|
_________ have weak conjugate bases that do not readily accept H+. As the strength of the acid decreases, the strength of its conjugate base increases. |
Strong acids |
|
|
In any acid–base reaction, there are two acids and two bases.However, |
one acid is stronger than the other acid, and one base is stronger than the other base. By comparing their relative strengths, we can determine the direction of the reaction. |
|
|
Because the dissociation of strong acids in water is essentially complete, the reaction is |
not considered to be an equilibrium process. Weak acids partially dissociate in water as the ion products reach equilibrium with the undissociated weak acid molecules. Formic acid is a weak acid that dissociates in water to form hydronium ion, H3O+, and formate ion, CHO2−. |
|
|
acid dissociation constant |
Ka - molar concentration of products divided by the molar concentration of the reactants - water is a pure liquid with a constant concentration and is omitted |
|
|
when value of Ka (acid dissociation ) is small |
the equilibrium lies to the left, favoring the reactants |
|
|
when value of Ka (acid dissociation ) is large |
the equilibrium lies to the right, favoring the products |
|
|
weak acids have _____Ka values |
small |
|
|
strong acids have ______Ka values |
large |
|
|
H3PO4 |
phosphoric acid - Ka = 7.5 x 10^-3 |
|
|
HNO2 |
nitrous acid - Ka = 4.5 x 10^-4 |
|
|
HF |
hydroflouric acid - Ka = 3.5 x 10^-4 |
|
|
HCHO2 |
formic acid - Ka = 1.8 x 10^-4 |
|
|
HC2H3O2 |
acetic acid - Ka = 1.8 x 10^-5 |
|
|
H2CO3 |
carbonic acid - Ka = 4.3 x 10^-7 |
|
|
H2S |
hydrosulfuric acid - Ka = 9.1 x 10^-8 |
|
|
H2PO4- |
dihydrogen phosphate - Ka = 6.2 x 10^-8 |
|
|
HCN |
hydrocyanic acid - Ka = 4.9 x 10^-10 |
|
|
HCO3- |
hydrgoen carbonate - Ka = 5.6 x 10^-11 |
|
|
HPO4^2- |
hydrogen phosphate - Ka = 2.2 x 10^-13 |
|
|
CH3-NH2 |
mehylamine - Ka = 4.4 x 10^-4 |
|
|
CO3^2- |
carbonate - Ka = 2.2 x 10^-4 |
|
|
NH3 |
ammonia - Ka = 1.8 x 10^-5 |
|
|
strong acids |
equilibrium position = toward products Ka = large conjugate base = weak |
|
|
weak acids |
equilibrium position = toward reactants Ka = small conjugate base = strong |
|
|
strong bases |
equilibrium position = toward products Ka = large conjugate base = weak |
|
|
weak bases |
equilibrium position = toward reactants Ka = small conjugate base = strong |
|
|
Water is amphoteric— |
it can act as an acid or a base. - In water, H+ is transferred from one H2O molecule to another. - one water molecule acts as an acid, while another acts as a base. - equilibrium is reached between the conjugate acid–base pairs. |
|
|
in pure water the concentrations of H3O+ and OH- at 25C are each |
1.0 x 10^7- M |
|
|
the ion product constant for water (Kw) is defined as |
- the product of the concentrations of H3O+ and OH- - equal to 1.0 x 10^14- at 25 C |
|
|
When [H3O+] and [OH−] are equal, the solution is |
neutral. |
|
|
when [H3O+] is greater than the [OH−], the solution is |
acidic. |
|
|
Kw= |
[H3O+] [OH−] |
|
|
[OH−] = |
Kw / [H3O+] |
|
|
[H3O+] = |
Kw / [OH−] |
|
|
In pure water, the ionization of water molecules produces |
small but equal quantities of H3O+ and OH− ions. - [H3O+] = 1.0 × 10−7 M - [OH−] = 1.0 × 10−7 M [H3O+] = [OH−] Pure water is neutral. |
|
|
adding an acid to pure water |
increases [H3O+] and causes [H3O+] to exceed 1.0 x 10 ^7- - decreases [OH−] |
|
|
[H3O+] > [OH−] The solution is |
acidic. |
|
|
Adding a base to pure water |
- increases the [OH−] - causes the [OH−] to exceed 1.0 × 10−7 M - decreases the [H3O+] |
|
|
[H3O+] < [OH−] The solution is |
basic. |
|
|
The pH scale is used to describe |
the acidity of solutions. A dipstick is used to measure the pH of urine. |
|
|
The pH of a solutionis used to indicate |
- the acidity of a solution. - has values that usually range from 0 to 14. - is acidic when the values are less than 7. - is neutral at a pH of 7. - is basic when the values are greater than 7. |
|
|
when [H3O+] > 1.0 X 10^-7 M |
acidic |
|
|
when [H3O+] < 1.0 X 10^-7 M |
basic |
|
|
The pH of a solution is commonly measured using chart. |
- a pH meter in the laboratory. - pH paper, an indicator that turns specific colors at a specific pH value. - The pH of a solution is found by comparing the colors of indicator paper to a |
|
|
The pH scale is a logarithmic scale that corresponds to the |
[H3O+] of aqueous solutions. - is the negative logarithm (base 10) of the [H3O+]. pH = −log[H3O+] - To calculate the pH, the negative powers of 10 in the molar concentrations are converted to positive numbers. If [H3O+] is 1.0 × 10−2 M, |
|
|
To determine the number of significant figures in the pH value, |
the number of decimal places in the pH value is the same as the number of significant figures in the coefficient of [H3O+]. the number to the left of the decimal point in the pH value is the power of 10. |
|
|
Because pH is a log scale,a change of one pH unit corresponds to a _______ change in [H3O+]. |
tenfold |
|
|
pH ________ as the [H3O+] increases. |
decreases |
|
|
pH 2.00 is |
[H3O+] = 1.0 × 10−2 M |
|
|
pH 3.00 is |
[H3O+] = 1.0 × 10−3 M |
|
|
Determine the [H3O+] for a solution that has a pH of 3.42. |
Enter the pH value into the inverse log equation and calculate. - 2nd, log, Ph value, +/- , = - 3.8 x 10^-4 is answer |
|
|
in neutralization reaction the salt formed is the |
the salt formed is the anion from the acid and the cation from the base. |
|
|
Antacids are substances that |
are used to neutralize excess stomach acid. are made of aluminum hydroxide and magnesium hydroxide mixtures. |
|
|
These hydroxides are not very soluble in water, so the levels of available OH− are not damaging to theintestinal tract. |
antacids |
|
|
basic compounds in antacids (hydroxides) |
Al(OH)3 = amphojel Mg (OH)2 = milk of magnesia Mg (OH)2 , Al(OH)3 = mylanta, mallox, digen,g elusin, riopan |
|
|
Titration is a laboratory procedure used to determine |
the molarity of an acid. uses a base such as NaOH to neutralize a measured volume of an acid. requires a few drops of an indicator such as phenolphthalein to identify the endpoint. |
|
|
The indicator phenolphthalein is added to |
identify the endpoint. turns pink when the solution is neutralized. |
|
|
At the endpoint of the titration, |
the moles of base are equal to the moles of acid in the solution. the concentration of the base is known. the volume of the base used to reach the endpoint is measured. the molarity of the acid is calculated using the neutralization equation for the reaction. |
|
|
A buffer solution maintains the pH by |
neutralizing small amounts of added acid or base. An acid must be present to react with any OH− added, and a base must be present to react with any H3O+ added. |
|
|
When an acid or a base is added to water, the pH |
changes drastically.. |
|
|
In a buffer solution, the pH is |
maintained; pH does not change when acids or bases are added |
|
|
Buffers work because |
they resist changes in pH from the addition of an acid or a base. in the body, they absorb H3O+ or OH− from foods and cellular processes to maintain pH. they are important in the proper functioning of cells and blood. they maintain a pH close to 7.4 in blood. |
|
|
A change in the pH of the blood affects the |
uptake of oxygen and cellular processes. |
|
|
A buffer solutioncontains a combination of |
acid–base conjugate pairs, a weak acid and a salt of its conjugate base, such as HC2H3O2(aq) and C2H3O2−(aq) - has equal concentrations of a weak acid and its salt. |
|
|
The pH of the solution is maintained as long as |
the added amounts of acid or base are small compared to the concentrations of the buffer components. |
|
|
calculating pH of a buffer |
rearranging Ka to give H3O+ - gives ration of acetic acid/ acetate buffer - products/ reactants - acid [H3O+] = Ka x acid/conjugate base - use [H3O+] to calculate pH - pH= -log [1.8 x 10 ^-5] |
|
|
Because Ka is a constant at a given temperature, = |
the [H3O+] is determined by the [HC2H3O2] / [C2H3O2−] ratio. - the addition of small amounts of either acid or base changes the ratio of [HC2H3O2]/[C2H3O2−] only slightly. - the changes in [H3O+] will be small and the pH will be maintained. the addition of a large amount of acid or base may exceed the buffering capacity of the system. |
|
|
Buffers can be prepared from conjugate acid–base pairs such as H2PO4−/HPO42− and HPO42−/PO43−, HCO3−/CO32−, or NH4+/NH3. |
The pH of the buffer solution will depend on the conjugate acid–base pair chosen. |
|
|
The arterial blood plasma has a normal pH of 7.35 to 7.45. If changes in H3O+ lower the pH below 6.8 or raise it above 8.0, cells cannot function properly and death may result. In our cells, CO2 |
- is continually produced as an end product of cellular metabolism. - is carried to the lungs for elimination, and the rest dissolves in body fluids such as plasma and saliva, forming carbonic acid, H2CO3. - As a weak acid, carbonic acid dissociates to give bicarbonate, HCO3−, and H3O+. |
|
|
To maintain the normal blood plasma pH (7.35 to 7.45), the ratio of [H2CO3]/[HCO3−] needs to be about |
1 to 10. concentrations of 0.0024 M H2CO3 and 0.024 M HCO3− work to maintain that pH. |
|
|
In the body, the concentration of carbonic acid is closely associated with |
the partial pressure of CO2, PCO2. |
|
|
If the CO2 level rises, increasing H2CO3, the equilibrium shifts to produce more |
H3O+, which lowers the pH. This condition is called acidosis. |
|
|
A lowering of the CO2 level leads to |
a high blood pH, a condition called alkalosis. |
|
|
respiratory acidosis |
CO2 increase, pH decrease - failure to ventilate, supression of breathing, disorientation, wekaness, coma |
|
|
metabolic acidosis |
H+ increase, pH decrease - increased ventilation, fatigue, confusion |
|
|
respiratory alkalosis |
CO2 decrease, pH increase - increased rate and depth of breathing, numbness, light headness, tetany |
|
|
metabolic alkalosis |
H+ decrease, pH increase - depressed breathing, apathy, confusion |
|
|
this is a H+ dono |
acid |
|
|
H+ donor that fully dissociates |
strong acid |
|
|
H+ donor that partially dissociates |
weak acid - Ka = [H3O+][A-] / [HA] |
|
|
H+ acceptor |
base |
|
|
H+ acceptor that partially dissociates |
weak base - Kb = [BH+][OH-] / [B] |
|
|
dissociation of H2O gives |
H2O and OH- |
|
|
dissociation of H2O giving H2O |
- log [H3O+] - THIS IS PH |
|
|
dissociation of H2O giving OH- |
kw= [H3O+] [OH-] |
|
|
acids and bases undergo neutralization to form |
water and salt of a weak acid or base with its conjugate forms a buffer to maintain pH - occurs in a titration and determines concentration of and Acid solution |
|
|
The primary difference between complete ionic and net ionic equations is |
the presence of spectator ions. Recall that spectator ions are those that do not participate in the reaction, and they can be identified as appearing on both sides of the reaction in equal amounts. |
|
|
When an acid or a base is added to a buffer, a |
double-displacement reaction will occur -The acid or base will be neutralized, forming a neutral salt or water plus the conjugate of the buffer |

