![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
162 Cards in this Set
- Front
- Back
|
define standard addition
|
known quantity of analyte is added to a specimen and the increase in signal is measured. The relative increase in signal allows us to infer how much analyte was in the origianal specimen.
|
|
|
What is the key asumption in standard addition
|
the signal is proportional to the concentration of analyte
|
|
|
When is standard addition used instead of a calibration curve
|
the sample matrix is complex or unknown (can't make a standard blood solution). Small volumes of concentrated standard are added to the unknown so that the matrix is not changed very much
|
|
|
What is the standarad addition equation
|
[Xi]/(Vo/V)[Xi]* [Sf]=Ix/Ix+s
express Xf as volume corrected ratio of Xi make sure to adjust volumes!! |
|
|
define internal standard
|
a known amount of a compond, different from analyte that is added to an unknown
|
|
|
when are internal standards useful
|
analyses in which the quantity of sample analyze or the insturment response varies slightly from run to run, the relative response between analyte and standard remains constant, also useful if sample is lost during preparation
|
|
|
what is the internal standard equation
|
area of analye/concentration of analyte= F* area of standard/ concentration of standard
|
|
|
what is oxidation
|
loss of electrons
|
|
|
what is reduction
|
gain of electrons
|
|
|
what is an oxidizing agent/ oxidant
|
takes electrons from another substance, becomes reduced
|
|
|
What is a reducing agent/ reductant
|
gives electrons to another substance, become s oxidized
|
|
|
how is electric charge (q) measured
|
couombs (C)
|
|
|
what does Faraday's constant represent
|
the number of coulmbs in a mole of electrons
|
|
|
T/F if we know how many electrons are transfered in a redox reaction, we can figure out how much product is formed
|
true. We can use Faraday's constant to figure out the number of moles of electrons transfered and then use molar ratios in the BALANCED redox reaction to figure out how many moles of product have been formed
|
|
|
what is current
|
measured in amperes, it is coulombs per second
|
|
|
what is a galvanic cell
|
a cell in which a spontanous chemical reaction that generates electricity, a battery
|
|
|
what is the purpose of a salt bridge
|
a bridge through which ions migrate in order to maintain electroneutrality in eah vessel
|
|
|
what would happend to a galvanic cell is there was no salt bridge
|
excess ions would build up creating an excess charge that would oppose the driving force for the chemical reaction, the process would cease
|
|
|
Where in a cell does reduction occcur? Where does oxidation occur?
|
reduction= cathode
oxidation= anode remember "An ox, red cat" |
|
|
when is the voltage of a galvanic cell positive
|
when the electrons are flowing into the negative terminal and out of the positive terminal (e- are moving towards more postive charge where they are spontaneously attracted)
|
|
|
what does the term "standard" imply when calculating a standard reduction potential
|
species are solids or liquids or their concentrations are 1M or their pressures are 1 bar, it is the difference between the standard potential of the reaction interest and the SHE
|
|
|
describe the SHE
|
catalyitic Pt surface in contact with an acidic solution in which [H+]= 1M, assigned potential is 0
|
|
|
Draw the line notation of SHE
|
Pt(s)| H2(g, 1 bar)| H+ (aq, 1M)||
|
|
|
How does the standard reduction potential relate to the spontaneity of a reaction
|
The more positive E°, the more favorable the reduction i.e. the more positive the standard reduction potential, the more a speices wants to be reduced. A reaction will be spontaneous when the speices with the more positive reduction potential is being reduced (E° is positive)
|
|
|
what are the values of E° and G for a spontaneous cell
|
E° is postive , G is negative
|
|
|
define formal potential
|
the potential of a cell containing a specified concentration of a reagent other than 1M
|
|
|
what species are omited from the nernst equation
|
pure solids, pure liquids, and solvents
|
|
|
if you need to multiply a half reaction to balance electrons, what should you do to E°
|
nothing. you do not multiply E°
|
|
|
How do you calculate Ecell
|
Ecell= E+-E-, or Ecath-Eanode or Erhs-Elhs, or Ere+Eox
|
|
|
in electrons flow towards more (positive/negative) potential
|
electrons flow towards more positive potential
|
|
|
log(x)-log(y)=
|
log(x/y)
|
|
|
describe how a cell equation can be used to calculate an equilibrium constant
|
by manipulating the nernst equation we arrive at E=E°-0.05916/n log Q
we know that at eqiulibrium E=0 and Q=K thus we can solve the equation for E° and then K K=10^nE°/0.05916 |
|
|
how does the sign of E° relate to K
|
if E° is positive K>1
if E° is negative K<1 if E° is zero K=1, the reaction is at equilibrium |
|
|
define reference electrod
|
maintains a constant potential
|
|
|
how do we arrive at the equation E=0.558 + 0.05916 log[Ag+] for the silver inidcator electrode
|
This equation takes into account that E° for silver is constant and the potential of SCE is constant. Both of these terms are combined into the 0.558. The +log term becomes positive because log(1/x)= -log(X)
|
|
|
HOw do we caulcuate the concentration of an ion in a potentionmetric precipitation titration before the equivalance point
|
Before the eqiuvalance point, we use the known excess of one ion and the ksp to find the concentration of the other
|
|
|
How do we calcuate the concentration of an ion in a potentionmetric precipitaiton titration at the equivalnce point
|
at the equivalence point, we know by definition that the concentration of the ions is identical thus we can take the square root of the ksp to solve for the concentration of the ions
|
|
|
How do we calculate the concentration of an ion in a potentiometric precipitation titration after the equivalence point
|
we have added a known excess of the titrant ion. [ion]= moles of excess/ total volume of solution
|
|
|
Why does the voltage barely change prior to the equivalance point of a potentiometric precipitaiton titration
|
the concentration of the titrant is very low and reltivley constant because it is being precipitated out of solution
|
|
|
what is the pupose of a double junction refrence electrode
|
it prevents titraiton error created by filling solution leaking out of the porus plug of the electrode. The double junction electrode has in inner electrode and an outer comparment filled with a solution that is compatible with the titration
|
|
|
when does a junction potential occur
|
when two dissimilar electrolyte solutions are placed in contact
|
|
|
what is a junction potential
|
a voltage difference that develops at the interface of two dissimilar electrolyte solutions due to the unequal mobility of the ions making up the solution
|
|
|
what is the consequence of junction potential
|
fundamental limitation on the accuracy of direction potentiometric measurements
|
|
|
why are relative potentiometric titration measurements more accurate than direct
|
relative measurements measure changes in potential observed during a titration, they are not affected by junction potential assuming it does not change between measurements
|
|
|
what is the purpose of the ligand "L" aka ionophore in the ion selective electrode
|
L selectivley binds the analyte cation (or anion). L is only soluble in the hydrophobic membrane
|
|
|
what is the purpose of the hydrophobic anion R- in an ion selective electrode
|
R- is only solube in the membrane so it cannot diffuse out into the solution. R- reversibly associates with cations, it provides the excess negative charge needed to establish the potential difference across the membrane
|
|
|
T/F there is redox chemistry employed when using an ion selective electrode
|
false. The potential across the membrane depends on the concentration of the analyte in the unknwon and the ability of the membrane to selectively bind the analyte of interest
|
|
|
describe the glass membrane of the pH electrode and how it respoinds to [H+]
|
-irregular network of SiO4
-Na+ ions move slowly through network -glass surface contains exposed O- that binds H+ from solution -H+ equilibrates with the glass surface giving the side of the membrane expose to a higher concentration of H+ a more positive charge |
|
|
how is a potential difference measure in a pH detecting glass electrode?
|
The potential difference is created when there is excess positive charge on one side of the membrane after H+ equilibrates with the exposed O-. Na+ ions in the glass carry electric current across the membrane (H+ ions do not move!)
|
|
|
what is the factor B in the response of glass electrode equation
|
B is the asymmetry potential, it corrects for the fact that the two sides of the membrane are not identical, it is very near 1
|
|
|
What are the 8 errors that can occur when measuring pH
|
1. Standards
2. junction potential 3. Junction potential drift 4. sodium error 5. acid error 6. equilibration time 7. hydration 6. temperature "The Jands" (temperature, hydration, equilibration, juction, acid, sodium (Na), drift, standards) |
|
|
How do standards contribute to error in pH measurement
|
Stanards are only accurate to +/- 0.01-0.02 pH units limiting the accuracy of our experimental measurements
|
|
|
How does junction potential contribute to error in pH measurement
|
Junction potential leads to an uncertainty of at least 0.01 pH units
|
|
|
How does junction potential drift contribute to errors in pH measurements
|
two effects contribute to a changing junction potential
1. AgCl can precipitate in the plug 2. If the analyte contains a reducing agent, Ag(s) can precipitate in the plug this error should be compensated for by recalibrating the electrode every 2 hours |
|
|
How does sodium error contribute to errors in pH measurements
|
When [H+] is very low and [Na+] is high, the electrode responds to Na+ as if it were H+.The apparent pH is lowe rthan the actual pH
|
|
|
how does acid error contribute to erros in pH measurement
|
in strong acid the measured pH is higher than the actual pH
|
|
|
describe how equilibration time leads to erros in pH measurements
|
in a well buffered solution with adequate stirring, equilibration of the glass with the analyte solution takes seconds. In a poorly buffered solition near the equivalence point of a titration, it could take minutes.
|
|
|
How does hydration contribute to errors in pH measurements
|
A dry electrode takes several hours in aqeuous soltion before it responds correctly to H+
|
|
|
How does temperature contribute to erros in pH measurement
|
pH meter must be calibrated at the same temperature at which the measurements will be made.
|
|
|
what is a selectivity coefficient
|
measures the extent to which an electrode responds to an interfering species. The smaller the selectivity coefficient, the less the interferance
|
|
|
define eludent
|
fluid entering the column
|
|
|
define eluate
define eluent |
eluent- fluid entering the column
eluate- fulid exiting the column (Remember, "ENTer" and "It's been aten" |
|
|
define elution
|
the process of passing liquid or gas through a chromotography column
|
|
|
Adsorption chromatography
stationary phase mobile phae mechansim |
stationary phase- solid
moblie phase- liquid or gas mechanism- solute is adsorbed on the surface of the solid particles |
|
|
Partition chromatography
stationary phase mobile phae mechansim |
stationary- thin liquid on solid support
mobile-liquid or gas mech-solute equilibrates between the stationary liquid and mobile phase |
|
|
Ion exchange chromatography
stationary phase mobile phae mechansim |
stationary-ionic groups covalently attached to solid, usually a resin
mobile-liquid mech-solute ions are atracted to the stationary phase by electrostatic forces |
|
|
molecular exclusion chromatography
stationary phase mobile phae mechansim |
stationary-pores small enough to exculde large molecules
mobile-liquid mech- separation by size. large particles stream past without entering pores but small ones take longer to pass through because they enter the pores and must flow throug ha larger volume before leaving the column |
|
|
affinity chromatography
stationary phase mobile phae mechansim |
stationary-molecule covalently attached to solid
mobile-liquid mechanism- highly specific, target in mobile phase binds to stationary phase, other components are washed out |
|
|
What are the axis of a chromatogram
|
detector response as a function of time or eleution volume
|
|
|
what is retention time
|
the time needed after injection for an induvidual solute to reach the detector
|
|
|
Describe the shape of an ideal chromatography peak
|
Gaussian shape. w1/2= 2.35 sigma, w at baseline is 4 sigma
|
|
|
How does bandwidth related to the number of theoretical plates in a column
|
The more theoretical plates, the more theoretical equilibration steps, the narrower the bandwidth (higher resolution)
|
|
|
What units is peak width measured in?
|
Time or volume (must be the same units as retention time)
|
|
|
What is the relationship between bandwit hand plate height
|
The smaller the plate height (more theoretical plates or a shorter colum H=L/N) the narrower the peaks. The ability of a column to separate components of a mixture is imporved by decreasing plate height
|
|
|
How is resolution defined
|
peak separation divided by average peak wideth
r=Δtr/Wav= 0.589Δtr/w1/2av |
|
|
what is the relationship between resolution and column length
|
resolution is proportional to the square root of column length. Doubling the length of a column will increase the resolution by √2
|
|
|
How can chromotography be used for qualitative analysis
|
-spiking on multiple stationary phases to see if the known sample increases the signal of the unknown (use two columns just in case the compouns are different but happend to have the sample retention time on a particular column)
|
|
|
How can chromotography be used for quantitiative analysis
|
-area of peak is proportional to quantity of analyte
-compare area of unknown to that of internal standard |
|
|
Describe the difference between an analytical and preparative column
|
analytical columns are long and thin to obtain good resolution
preparative columns are short and fat so that larger quantities can be handled |
|
|
How can column be scaled up? What shold be kept constant? What should be increased proportionally.?
|
scaling equation
large load/small load= large radius^2/small radius ^2 -maintain a constant ratio of unknown to column volume by keeping the lenght the same and increasing the cross sectional area -the volume flow rate should be increase in proportion to the cross sectional area (if area is 10x bigger, flow should be 10x faster) |
|
|
What are the three factors that contribute to band broadening
|
1. diffusion
2. slow equilibration 3. multiple paths |
|
|
Describe the relationship between bandwidth and flow rate in terms of diffusion
|
-The farther a band has travled, the more time it has to diffuse and the broader it becomes
-The faster the flow rate, the less time a band spends in the column and the less time ther is for diffusion to occur -thus, boradening by longitudinal diffusion is inversely proportional to flow rate |
|
|
Describe the relationship between bandwidth and flow rate in terms of equilibration time
|
the solute requires time to equilibrate between the phaeses, if this does not happen rapidly, solute in the stationary phase lags behind that in the mobile phase
-the faster the flow rate, the broader the peaks -broadening due to finite rate of mass transfer is directly related to flow rate |
|
|
What affect does temperature have on resolution?
|
Raising the temperature increases the rate of mass transfer which lessens the effect of broadening with faster flow rates
|
|
|
Describe the relatioship of multiple paths to peak brodening
|
Some random flow paths are longer than others so some of the solute may remain in the column longer. This property is not related to flow rate
|
|
|
How might bands broaden outside of a chromatography column
|
-too much tubing
-detector has too large of a volumne |
|
|
Describe an open tubular column (capillary tube)
|
-hollow tube whose inner wall is coated with a thin layer of stationary phase
|
|
|
What are the benefits of using a caplillary column over a packed column
|
-no broadening from multiple paths so better resolution (more theoretical plates)
-can be made much longer with same operating pressure, greater length and smaller plate height give much better resoultion |
|
|
What are the disadvantages of using a capillary column over a packed column
|
open tubular column cannot handle as much solute as a packed column because there is less stationary phase. Tubular columns are useful for analytical sepractions but not preparative
|
|
|
describe overloading and its affect on peak shape
|
-overloading occurs when there is too much solute in one band
-this occurs because the compound is most soluble in itself, the solulte is so soluble in teh concentrated part of the band that little trails behind -this leads to a curve with an abrupt cut off after the peak |
|
|
Describe tailing and its affect on peak shape
|
-tailing occurs when there are strongly polar, highly adsorptive sites in the stationary phase that retain solute more strongly than other sites
-leads to a cure with more solute coming out after the peak retention time -can be reduced by silanization to convert polar OH groups to non polar OSi(CH3)3 groups -increased tailing indicates that the column needs to be replaced |
|
|
what is the relationship between vapor pressure and elution time
|
the higher the vapor pressure the faster the compound is eleuted
|
|
|
What is the purposeof temperature programming?
|
-used to separate compounds with a wide reandge of boiling points or polariteis
-column temp is raised during the separation to decrease retention time and sharpen peaks |
|
|
what is the rule to follow when choosing a statinoary phase for GC?
|
like dissolves like
-nonplar colums are best for nonpolar solutes -polar columns are best for polar solutes |
|
|
Why are H2 and He better carrier gases (give better resoultion ) than N2
|
Solutes diffuse more rapidly through H2 and He and therefore equilibrate between the mobile and stationary phase more rapidly
|
|
|
How does a flame ionziation detector work
|
-eluate is burned in a mixture of H2 and air
-carbon atoms (except carboxyl and carbonyl) produce CH radicals which produce CHO+ and an electron in the flame -CH+O=> CHO+ + e- -the flow of ions and electrond to the electrodes produces the detector signal -the detector signal is proportional to the number of susceptible carbon atoms entering the flame |
|
|
Describe how a thermal conductivity detector works
|
-measures the ability of a substance to transport heat
-when solute emerges from the column the thermal conductivity of the gas stream decreases, the filament gets hotter, resistance increase and voltage across it increases -the voltage change is the detector signal |
|
|
Describe how an electron capture detector works and what it is sensitive to
|
-sensitive to halogen containing molecules
-insensitive to hydrocarbons, alcohols, ketnoes -carrier gas is ionized by high energy electrons -electrons liberated from the gas are attracted to an anode and produce a current -when analyte molecules of high electron affinity enter the detector, they capture electrons and reduce the current -the detector respons by varying the frequency of voltage pulses to maintain a constant current |
|
|
Describe how a mass spectrometry detector works
|
-most versatile detector
-ions separated based on m/z ratio |
|
|
What are the essential compoents of an HPLC insturment
|
-solvent delivery system
-sample injection valve -detector -computer to control system and display results |
|
|
What is the relationship between stationary phase particle size and resolution in HPLC
|
resolution increases as stataionary phase particle size decreases
|
|
|
What is the basic mechanism of HPLC
|
high pressure is used to force eluent through a colsed column packed with micrometer size particles
|
|
|
What is normal phase chromatography? How is eluent strength increased?
|
-polar stationary phase
-less polar solvent Eluent strength is increased by adding a more polar solvent |
|
|
What is reversed phase chromatography? How is eluent strength increased?
|
-non polar stationary phase
-more polar solvent -eluent strength is increased by adding a less polar solvent |
|
|
What is most commonly used in HPLC as a stationary support? What is this molecules polarity
|
Silica is used, it is polar.
The particles are permeable to solvent and have a large surface area, solute adsorption occurs directly on the silica surface |
|
|
What is the purpose of octadecyl silane in HPLC
|
octadecyl silane is a non polar bonded stationary phase covalently attacned to silanol groups on the silica surface
|
|
|
What is the difference between isocratic and gradient eluction
|
isocratic-elution with a single solvent or constant solvent mixture
gradient-solvent is changed continuously from weak to stronger eluent strength my mixing more strong solvent into weak solvent during the separation |
|
|
What is the purpose of octadecyl silane in HPLC
|
octadecyl silane is a non polar bonded stationary phase covalently attacned to silanol groups on the silica surface
|
|
|
What is the difference between isocratic and gradient eluction
|
isocratic-elution with a single solvent or constant solvent mixture
gradient-solvent is changed continuously from weak to stronger eluent strength my mixing more strong solvent into weak solvent during the separation the stronger the eluent strength, the more the mobile phase can pull the solute off the stationary phase |
|
|
define chemical oxygen demand
|
measure of the amount of chemicals (usually organic) that consume dissolved oxygen
|
|
|
Why must a back titration be used in the COD experiment?
|
-the initial reaction is very slow
-the end point of the initial reaction is not very clear |
|
|
Describe the "titration scheme" of the COD experiment
|
A known excess of dichromate is added to the sample containing an unknown amount of oyxgen. After the reaction, the reamining dichromate is titrated with the standard iron solution to figure out how much dichromate was used. We can then related the amount of dichromate consumed to the chemical oxygen demand of the sample.
|
|
|
Write the two balanced titration reactions for the COD lab
|
Back titration
6Fe 2+ +Cr2O7 2- + 14 H+ => 2 Cr2+ + 7H2O +6 Fe3+ (6 moles Fe for 1 mol dichromate) initial reaction 4Cr2O72- +8 H2O+ 56H+ => 8Cr3+ + 28H2O+ 6O2 |
|
|
What is the indicator used in the back titration of COD lab? Describe the chemistry and color change that occurs
|
The indicator is ferroin aka Fe(II) orthophenanthroline
The Fe2+ (blue-green) is oxidized by dichromate to Fe3+ (brown-red) (remember reduced is red) |
|
|
Explain how the chemical oxygen demand of a sample was calculated in the COD lab
|
From the back titration we converted moles of ferrous ammonium sulfate hexahydrate to moles to dichromate (1 mol dichromate/6 mol Fe) this tells us the excess moles of dichromate
we subtract this from the original moles of dichromate to get the moles of dicrhomate consumed in the reaction -we convert moles of dicrhomate to moles of oxygen (3 moles to 2 moles dichromate -then we express Mol/L dichromate as mg O2/L solution |
|
|
How is COD expressed
|
mg O2/L necessary to carry out the oxidation of the sample to CO2 and H2O
|
|
|
What are the five essential requirements of an ISE
|
1. selectivity
2. fast response 3. durable and not easily contaminated 4. reproducible results 5. simple to construct and use |
|
|
What is the reference electrode used in the fluoride electrode lab
|
Ag (s)| AgCl(s)|KCl, (1M, aq)||
|
|
|
What is the chemical name and formula of the fluoride ISE
|
the crystal is made from LaF3 (lanthanum floride) doped with Eu2+
|
|
|
What are delta C and S in the flouride ISE equation
|
delta C- the number of MOLES of standard fluoride added
S-Nernst factor (2.303 RT/nF) |
|
|
How is K calculated in the Silver electrode lab
|
K=Ecell +Nlog (1/A Ag+)
K is just a combination of all of the constants including the standard potential of silver and the reference electrode N is the nernst factor (2.303 RT/nF) |
|
|
How is the activity of silver calculated from the cell potential in the silver electrode lab
|
A Ag+= 10^ (Emeasured-K/N)
|
|
|
What type of detector is used in the GC from lab and how does it work?
|
Thermal conductivity
The detector is a filament which is heated electrically. When pure carrier gas flows through the detector, the temperature of the filament remains constant. WHen a sample peak elutes, the thermal conductivity of the gas changes and causes a change in filament temperature. If the analyte decreases the thermal conductivity, the filiment will heat up which will change its resistance. The change in resistance is converted to a chante in voltage which is plotted as a function of time. |
|
|
Why is the internal standard method used in GC analysis
|
Small fluctucations in temperature make it difficult to obtain a straight line from the calibration curve. It is more acurate to determine the response factor using an internal standard because it is subjected to the exact same conditions as the analyte
|
|
|
What is the chemical name of the internal standard used in the GC lab?
|
n-butyl alcohol
|
|
|
What is the stationary and mobile phase used in the HPLC lab
|
statinoary- octadecyl groups chemcially bounded to porous silica microspheres
mobile- 50/50 MeOH and H2O |
|
|
What were the three compounds analysed in the HPLC lab and what were their retention times
|
caffiene- 176 s
theophylline- 144 s theobromine 126 s |
|
|
What is the equation used to calculate the concentration of analyte in the unknown in the HPLC lab?
|
[analyte] in unknown =
(unknown peak*(volume std/total volume) * [standard])/ (mixture peak-[(unknown peak * volume unknown)/ total volume]) |
|
|
Draw the structure of EDTA and give its full name
|
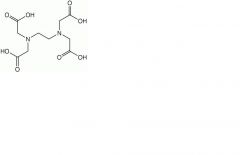
Ethylenediaminetetraacetic acid
|
|
|
What is the stoichimetry of the titration reaction in the hardness of water lab
|
1:1 EDTA makes 1:1 complex with Calcium
there is 1 mol of Ca for every mol of CaCO3 |
|
|
What is the pupose of calmagite in the hardness of water lab?
|
calmagite is the indicator. Initially, Calmagite is bound to Mg-In- (red). When all of the Ca has been used up, EDTA then displaces the Mg from the In leaving free In. The In 3- then is protonated to give a blue colored HIn2-
|
|
|
What color change is observed in the hardness of water titration
|
the calmagite indicator changes from the red Mg-In- to the blue HIn2-
|
|
|
What is the purpose of the Mg(II) EDTA in the hardness of water lab
|
to shpared the endpoint of the titration
|
|
|
What is the purpose of the ammonia buffer in the hardness of water lab
|
1. keeps the pH high enough so that EDTA is in the Y4- form
2. auxillary complexing agent that prevents the precipitation of metal hydroxides |
|
|
What is the definition of hard water and how are hard water values reported
|
Hard water refers to water containing a significant amount of cations. Hardness is numerically expressed in ppm CaCO3. <40ppm is soft, >150 ppm is hard
|
|
|
How is hardness of water calculated from an EDTA titration
|
Stardardized ETDA is added to the unknown sample. The EDTA reacts 1:1 with calcium so you can determine the moles of calcium in the water. From this you can figure out the grams/L of CaCO3 (1 mol Ca for 1 mol CaCO3) and subsequently the ppm CaCO3
|
|
|
Draw a diagram of a GC chromatograph
|
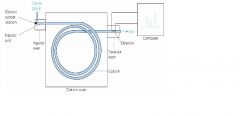
|
|
|
What two parameters of the Van Deemter equation change in a capillary vs packed column that give the capillary better resolution
|
There are not multiple paths so A=O. The rate of equilbration is faster so C is smaller
|
|
|
How does the ration of [stationary phase]/[mobile phase] change when overloading occurs
|
The concentration of solute in the mobile phase increases. This bends a liner graph of Cs/Cm upwards
|
|
|
How does the ration of Cs/Cm change when tailing occurs
|
The concentration of solute in the stationary phase increases. This bends a linera graph of Cs/Cm downwards
|
|
|
Is chromatography good for qualititative anaylsis
|
no but you can use a mass spec detector to do this
|
|
|
What are the key parts of an HPLC insturment
|
injection port, column, mobile phase reservoir, detector, computer
|
|
|
Describe what it means to be a monodentate or multidentate ligand
|
monodentate-a ligand that binds to a metal through only one atom
multidentate-a ligand that binds to a metal through more than one atom. EDTA is hexadenate because it binds to a metal through 2 N atoms and 4 O atoms a multidentate ligand is also called a chelating ligand |
|
|
Describe the bonding strength to EDTA of a metal ion indicator
|
it must bind the metal less strongly than EDTA to be useful , the metal will leave the indicator in favor of complexing with EDTA, the free indicator is a different color than the metal bound form
|
|
|
Describe a displacement titration
|
the analyte is treated with excess Mg(EDTA)2- to displace Mg
-the analyte metal complexs with the added EDTA -the left Mg is then titration with standard EDTA while |
|
|
What is the purpose of a masking agent
|
protects some component of the analyte from reacting with EDTA. Use when you only want to titrate one metal in a solution with multiple metals present
|
|
|
what is the purpose of using a conditional formation constant
|
the conditional formation constant describe the formation constand of MYn-4 at any particular pH. It allows us to look at EDTA complex formation as if the uncomplexed EDTA were all in one form
|
|
|
How is the concentration of free metal calculated before the equivalnace point of an EDTA titration
|
the concentration of free metal is equal to the concentration of excess uncreated mental. Calucluate the original mmol of metal present and subtract the number of mmol of EDTA added. Divided the remaining mmol of metal by the new totoal volume. THe amount of free metal coming from the dissociation of the complex is negligible
|
|
|
How is the concentration of free metal calculated at the equivalance point of an EDTA titration
|
There is exactly as much EDTA as metal in the solution which we set equal to a concentration of X M. The concentration of complex is equal to the formal concentration of the complex (which is calculated by finding the number of mmol of metal originall present and assuming it is now all complex) minus the amount that dissociates.
From this we arrive at: K'f= F-x/x^2 |
|
|
How is the concentration of free metal calculated after the equivalance point of an EDTA titration
|
-we know that the concentration of EDTA is just the volume excess * molarity/ new total volume
-the concentration of complex is the formal concentration (the mmol of metal originally present is now all complex) dividied by the new total volume -using the K'f equation, we can solve for the concentration of free metal |
|
|
What is plotted in an EDTA titration curve
|
pMetal on the Y axis, volume EDTA on the X
|
|
|
What are delta G, delta E, and Q vs. K for a spontaneous cell?
|
delta G is negative
delta E is positive Q<K (product favored) |
|
|
What are the delta G, delta E and Q vs K for a nonspontaneous reaction
|
delta G is positive
delta E is negtaive Q>K (reactant favored) |
|
|
Derive the Nerst equation
|
ΔG=-nFE
ΔG=ΔGº +RTlnQ -nFE=-NFEº +RTlnQ (divide both sides by -nF) E=Eº- RTlnQ/nF At 25º we can simplify RT/nF and turn the ln to log (ln10=2.303) to arrive at E=Eº-0.05916/n log Q |
|
|
How can we use electrochemistry to find the K of a reaction
|
1. find 2 half reactions that add to the overall reaction for K
2. at equilibrium we know that Ecell=0 and Q=K |
|
|
How do you calcuate the cell potential with a reference and indicator electrode
|
Ecell= Eindicator-E reference+ E junction
|
|
|
How is Ecell caluculated for an ion selective electrode
|
Emeas=Eref1-Eref2+Ejunction+E bound
|
|
|
T'rA/T'rB is proportional to
|
Ka/Kb retention time is proportinal to equilibration between stationary and mobile phase
|
|
|
H=L/N what is another way to express thsi
|
H=sigma^2/x, the variance of the band over the distance the band travelled
|
|
|
describe resolved peaks in terms of gaussian curves
|
-The gausain curves have a width of 4 sigma (=/- two sigma from the mean, ignoring the very edge)
-The change in retention time (middle of the curve) is 6 sigma (includes the edge) -resolution is Tr/wav or 6/4 which is 1.5 -asume that only one side of the area outside of 3 sigma overlaps -3 sigma is 99.7% of the curve, so 0.3% remains, divide this by two because you're only looking at 1 side ant you get a 0.15% overlap |
|
|
Describe the stationary phase used in the HPLC experiment
|
octadecyl silane, modified silica particles with large surface area, non polar
|
|
|
What factors make up the constant used in the fluoride ion electrode lab
|
1. reference electrode 1
2. reference electrode 2 3. junction potential 4. Activity of Fluoride in the known solution 5. activity coefficient of the unknown solution |

