![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
|
Ionic Reactions:
|
those in which covalent bonds break heterolytically & in which ions are involved as reactants, intermediates, or products
|
|
|
A general homolysis:
|

|
|
|
What must be supplied to cause homolysis of covalent bonds? In what 2 ways is it done?
|
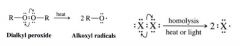
Energy must be supplied in the form of
1. heating 2. irradiation w/ light. |
|
|
Hydrogen Abstraction:
|
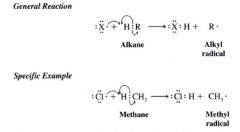
|
|
|
When atoms combine to form molecules, what is released as covalent bonds form?
|
energy (the molecules of the products have lower enthalpy than the separate atoms)
|
|
|
Bond formation is what type of process?
|
exothermic
|
|
|
energy must be supplied to do what with the bonds?
|
break covalent bonds
|
|
|
reactions in which only bond breaking occurs are always what type of process?
|
endothermic
|
|
|
Homolytic bond dissociation energies:
|
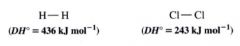
energies required to break covalent bonds homolytically
|
|
|
Table 10.1: Single-Bond Homolytic Dissociation Energites DH @ 25 degrees C.
|

|
|
|
For bond breaking delta H is ____(1)____, and for bonds forming delta H is ___(2)_____
|
1: positive
2. negative |
|
|
Energy diagram showing stability of primary, secondary, tertiary radicals:
|
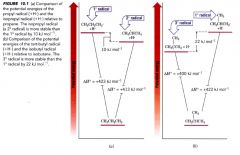
|
|
|
The general stability of alkyl radicals:
|

|
|
|
Halogenation:
|

- substitution reaction of an alkane w/ a halogen
- a halogen atom replaces 1/ more of the H atoms of the alkane. (methane, ethane, and other alkanes react w/ the first 3 members of the halogen family: F, Cl, Br... alkanes do not react appreciably w/ I) |
|
|
Why does multiple substitution reactions almost always occur?
|
b/c all H atoms attached to C are capable of reacting w/ F, Cl, or Br.
|
|

|

|
|
|
Elaborate on the statement "chlorination is unselective"
|
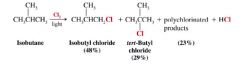
Chlorination of most higher alkanes gives a mixture of isomeric monochloro products as well as mroe highly halogenated compounds.
- Cl is relatively unselective, meaning it does not discriminate greatly among the different types of H atoms (primary, secondary, tertiary) in an alkane. |
|
|
Explain an exception in the halogenation of an alkane (or cycloalkane) whose H atoms are all equivalent?
|
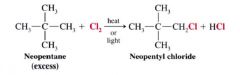
Neopentane
- it can form only one monohalogenation product, and the use of a large excess of neopentane minimizes polychlorination. |
|
|
The Chlorination of Methane:
|
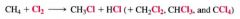
1. the reaction is promoted by heat or light.
2. the light-promoted reaction is highly efficient. |
|
|
A Mechanism for the reaction: Radical Chlorination of Methane:
|

|
|
|
Describe each step in a chain reaction:
|
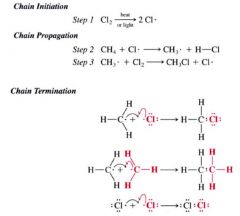
1. Initiation step (radicals are created)
2. propagation step (one radical generates another) 3. Termination step (basically all possible radials combine) |
|
|
Describe each step in a chain reaction:
|

1. Initiation step (radicals are created)
2. propagation step (one radical generates another) 3. Termination step (basically all possible radials combine) |
|
|
Entropy:
|
measures the relative disorder/ randomness of a system
|
|
|
For a chemical system, entropy of the molecules can be related to what? and what it associated with?
|
related to the # of degrees of freedom available to the molecules & their constituent atoms.
- degrees of freedom is associated w/ ways in which movement/ changes in relative position can occur. |
|
|
What are the 3 degrees of freedom in a molecule?
|

1. Translational: associated w/ movements of the whole molecule through space
2. Rotational: associated w/ the tumbling motions of the molecule 3. vibrational: associated w/ the stretching & bending motion of atoms about the bonds that connect them |
|
|
If the atoms of the products of a reaction have more degrees of freedom available than they did as reactants, the entropy change (delta S) for the reaction will be?
|
positive
|
|
|
atoms of the products that are more constrained (have fewer degrees of freedom) than the reactants, has a _________ entropy change (delta S).
|
negative results
|
|
|
a low energy of activation means a reaction will take place ________?
|
rapidly
|
|
|
a high energy of activation means that a reaction will take place ________?
|
slowly
|
|
|
Energy of activation must be determined from experimental data and can not be directly measured (it is calculated) What are certain principles that have been established that enable one to arrive at estimates of energies of activation:
|

|
|
|
Thermal cracking:
|
when a compound is heated to a very high temperature, radical reactions take place that produce other products.
|
|
|
What does the Energy of activation for a reaction equal to delta H mean?
|
that bonds are broken homolytically but no bonds are formed.
|
|
|
What does the Energy of activation for a reaction greater than zero mean?
|
covalent bonds are broken.
|
|
|
What does the Energy of activation for a reaction equal to zero mean?
|
these reactions are gas phase reactions in which small radicals combine to form molecules.
|
|
|
What is the observed order of reactivities in halogenation?
|
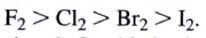
the less reactive, the higher the energy of activation meaning that only a very small fraction of all of the collisions will be energetically effective.
|
|
|
A Mechanism for the Reaction: Radical Halogenation of ethane:
|

|
|
|
What is the correlation between reactivity of different H atoms and the type of H atoms (primary, secondary, tertiary) being replaced?
|
The tertiary H atoms of an alkane are most reactive, secondary H atom are next most reactive, and primary H atoms are the least reactive
(note: primary H atoms are attached to primary C atoms.. and so forth) |
|
|
What is the geometric structure of most alkyl radicals?
|

trigonal planar at the carbon having the unpaired e-s.
- sp2 hybridized central Carbon. |
|
|
When achrial molecule react to produce a comound w/ a single tetrahedral chirality center, the product will be obtained as a________?
|
racemic form.
|
|
|
A Mechanism for the reaction: The Stereochemistry of Chlorination at C2 of Pentane:
|

|
|
|
A Mechanism for the Reaction: The Stereochemistry of Chlorination at C3 of (s)-2-Chloropentane:
|
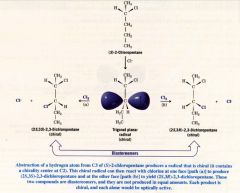
|
|
|
What was the cause of getting @ times Markovnikov addition & @ other times getting Anti-markovnikov under the same conditions?
|
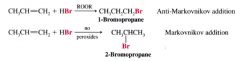
Turned out it was due to the presence of organic peroxides in the alkenes... peroxides were formed by the action of atmospheric oxygen on the alkenes
- alkenes that contain peroxides (ROOR) or hydroperoxides (ROOH) reacted w/ HBr... ---- anti-markovnikov addition of HBr occured - Is a radical chain reaction initiated by peroxides. |
|
|
A Mechanism for the Reaction: Anti-Markovnikov Addition of HBr.
|

|
|
|
Describe Markovnikov addition of HBr:
|
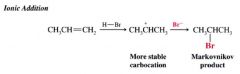
the reagent that attacks the double bond first is a proton. Because a proton is small, steric effects are unimportant. It attaches itself to a carbon atom by an ionic mechanism so as to form the more stable carbocation.
|
|
|
What type of solvents favor Markovnikov addition of HBr?
|
protic and polar solvents.
|
|
|
Does HF, HCl, HI give anit-markovnikov in the presence of peroxides (ROOR)?
|
No
|
|
|
Describe the anti-Markovnikov addition of HBr:
|

- occurs in the presence of peroxides
- the reagent attacks the double bond first is the larger bromine atom. - it attaches itself to the less hindered carbon atom by a radical mechanism, so as to form the more stable radical intermediate. |
|
|
What solvents are preferable in an anti-Markovnikov addition of HBr?
|
nonpolar solvents
|
|
|
Polymerizations:
|
reactions by which monomers are joined together
|
|
|
A Mechansim for the Reaction: Radical Polymerization of Ethene:
|

|
|
|
Acid-catalyzed polymerization:
|
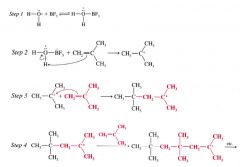
the polymerization of alkenes when they are treated w/ strong acids.
The growing chains are cations rather than radicals. The catalysts used for cationic polymerizations are usually lewis acids that contain a small amount of water. |
|
|
Concept Map: Mechanism Review of Radical Reactions:
|

|
|
|
Acid-catalyzed polymerization:
|
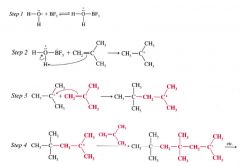
the polymerization of alkenes when they are treated w/ strong acids.
The growing chains are cations rather than radicals. The catalysts used for cationic polymerizations are usually lewis acids that contain a small amount of water. |

