![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
29 Cards in this Set
- Front
- Back
|
Chirality/ Chiral:
|
- An object that possesses the property of "handedness"
- one that cannot be placed on its mirror image so that all parts coincide - not super posable |
|
|
Isomers:
|
different compounds that have the same molecular formula.
|
|
|
constitutional isomers:
|
have the same molecular formula, different connectivity
- their atoms are connected in a different order |
|
|
stereoisomers:
|
- not constitutional isomers
- have their atoms connected in the same sequence, but differ in the arrangement of their atoms in space |
|
|
enantiomers:
|
stereoisomers whose molecules are nonsuperposable mirror images of each other.
|
|
|
diastereomers:`
|
are stereoisomers whose molecules are not mirror images of each other.
|
|
|
Chiral molecule:
|

molecule not superposable on its mirror image
|
|
|
enantiomeric:
|
The relationship between a pair of enantiomers
|
|
|
achiral:
|
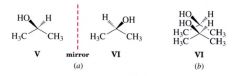
molecules that are superposable on their mirror image
|
|
|
flow chart on the subdivision of isomers
|
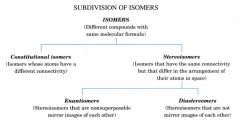
|
|
|
Stereogenic center:
|
an atom @ which an iterchagne of groups produces a stereoisomer.
- interchanging any two groups at the chriality center converts one enantiomer into the other. |
|
|
Plane of symmetry:
|
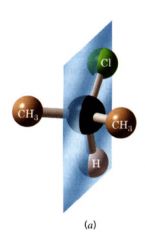
(mirror plane)
an imaginary plane that bisects a molecule in such a way that the two halves of the molecule are mirror images of each other. |
|
|
Assigning R & S configuration:
|
1. eqach of the 4 gorups attached to the chirality center is assigned a priority/ preference a,b,c,d.
--- assigned on the basis of the atomic # of the atom that is directly attached to the chirality center (lowest atomic # = lowest priority) 2. When a priority can not be assigned on teh basis of the atomic # that is directly attached to the chirality center, then the next set of atoms in the unsigned group is examined 3. Rotate so group w/ lowest priority is directed away 4. if priority runs clockwise from highest to lowest the enantiomer is designed (R); counter clockwise is designed (S). 5. Groups containing double/ triple bonds are assigned priorities as if both atoms were duplicated/ triplicated. |
|
|
dextrorotatory (+):
|
substance that rotates plane- polarized light in the clockwise direction
|
|
|
levorotatory (-):
|
substance that rotates plane-polorized light in a counterclockwise direction
|
|
|
Specific rotation:
|

measured rotation on a standard basis.
|
|
|
Racemic mixture:
|
- an equimolar mixture of 2 enantiomers
- causes no net rotation of plane-polarized light. |
|
|
Calculating enantiomeric excess (ee)/ optical purity:
|

|
|
|
Stereoselective reactions:
|
reactions that lead to a preferential formation of the stereoisomer over other stereisomers that could possibly be formed.
|
|
|
Enantioseelctive:
|
if a reaction produces preferentially one enatiomer over its mirror image
|
|
|
diastereoselective:
|
if a reaction leads preferentially one diasteromer over others that are possible.
|
|
|
enzymes:
|
- Biological catalysts of extraordinary efficiency
- in nature what causes most stereoselective reactions - have the ability to cause reactions to take place much more rapidly than they would otherwise and have the ability to assert a dramatic chiral influence on a reaction |
|
|
Meso Compound:
|

compounds that are not chiral even though it contains two chirality centers
- b/c they are achiral... optically inactive. |
|
|
naming compounds with more than one chirality center:
|
if a compound has more than one chrality center, we analyze each center separately and decide whether it is (R) or (S). Then using numbers, we tell which designation refers to which carbon atom.
|
|
|
Fisher projection formula:
|
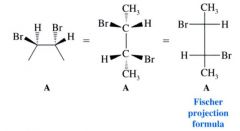
first it is necessary to note that in Fisher projections the carbon chain is always drawn from top to bottom, and also consider the molecule in a conformation that has eclipsing interactions between the groups at each carbon.
|
|
|
If a compound is not chiral, is it optically acaitve?
|
no
|
|
|
What does it mean when say "what is the absolute configuration"?
|
it means the configuration is including the R and or S configuration.
|
|
|
How are enantiomers separated?
|
1. allowing a racemic form to react with a single enantiomer of some other compound.
--> this changes a racemic form into a mixture of diastereomers (have different m.p., b.p., solubilities...) therefore can be separated by conventional means. |
|
|
atropisomers:
|
conformational isomers that are stable, isolable compounds.
|

