![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
Radioactivity |
The emission of subatomic particles or high energy electromagnetic radiation by the nuclei of certain atoms. |
|
|
Types of Radioactivity |
Alpha Decay Beta Decay Gamma Ray Emission Positron Emission Electron Capture |
|
|
Alpha Decay |
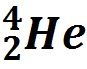
Unstable nucleus emits a particle composed of 2 protons and 2 neutrons. |
|
|
Beta Decay |
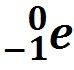
Unstable nucleus emits an electron. |
|
|
Gamma Ray Emission |
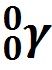
Gamma ray photon emitted with an alpha particle. |
|
|
Positron Emission |
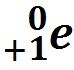
The antiparticle of an electron |
|
|
Electron Capture |
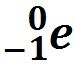
Involves an electron being absorbed instead of emitted. |
|
|
Ionizing Power |
Ability of radiation to ionize molecules and atoms. Alpha>Beta=Positron>Gamma |
|
|
Penetrating Power |
Ability of radiation to penetrate matter Gamma>Beta=Positron>Alpha |

