![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
61 Cards in this Set
- Front
- Back
|
Thermochemistry:
|
The study of the relationships between chemistry and energy.
|
|
|
Energy:
|
the capacity to do work
|
|
|
Work:
|
the result of a force acting through a distance.
|
|
|
Heat:
|
the flow of energy caused by a temperature difference.
|
|
|
example of energy and work:
|

|
|
|
Kinetic Energy:
|
energy associated with the motion of an object.
|
|
|
Thermal Energy:
|
energy associated with the temperature of an object. (also a form of kinetic energy)
|
|
|
Potential Energy:
|
energy associated with the position / composition of an object.
|
|
|
Chemical Energy:
|
the energy associated with the relative positions of electrons and nuclei in atoms and molecules (also a form of potential energy).
|
|
|
ENERGY TRANSFORMATION 1: energy transformation: potential and kinetic energy:
|

|
|
|
The Law of Conservation of Energy:
|
energy can be neither created nor destroyed. However, energy can be transferred from one object to another, and it can assume different forms.
|
|
|
Energy Transformation: Potential and Kinetic Energy
|

|
|
|
Energy Transfer
|

|
|
|
KE =
|

|
|
|
Joule (J):
|
named after the english scientist James Joule (1818-1889)
- relatively small amount of energy |
|
|
1 J =
|

|
|
|
calorie (cal):
|
originally defined as the amount of energy required to raise the temperature of 1g of water by 1 degree C.
1 cal = 4.184J |
|
|
Energy Conversion Factors:
|

|
|
|
Energy Uses in Various Units:
|

|
|
|
Thermodynamics:
|
the general study of energy and its interconversion
|
|
|
first law of thermodynamics:
|
- the total energy of the universe is constant.
- because energy is neither created nor destroyed, and the universe does not exchange energy with anything else, its energy constant does not change. |
|
|
Internal Energy (E):
|
the sum of the kinetic and potential energies of all of the particles that compose the system
- is a state function. |
|
|
State Function:
|

its value depends only on the state of the system, not how the system arrived at that state.
|
|

|

|
|
|
Where does the energy lost by the reactants in a reaction go?
|

energy must flow out of the system (reactant & products of the reaction) into the surroundings.
|
|
|
The internal Energy change of the system (delta E):
|
q + w
(heat + work) |
|
|
Sign Conversions for q, w, and delta E
|

|
|
|
FIG 6.4: Energy, Work, and Heat
|

|
|
|
Temperature:
|
a measure of the thermal energy of a sample of matter
|
|
|
Thermal energy always flows from matter at _________ to matter at _________
|
higher temperature, lower temperature.
ex: a hot cup of coffee transfers thermal energy ---as heat--- to the lower temp. surroundings as it cools down. |
|
|
Thermal Equilibrium:
|
- no additional net transfer of heat.
- the object and surroundings reach the same temp. |
|
|
When a system absorbs heat (q) its temperature changes by delta T:
|

|
|
|
heat capacity (C):
|
- the constant of proportionality between q and delta T.
- a measure of the system's ability to absorb thermal energy without undergoing a large change in temperature. - the quantity of heat required to change the temp. by 1 degree C. - is an extensive property-- depends on the amount of matter being heated. |
|
|
specific heat capacity:
|
The amount of heat required to raise the temp. of 1 g. of the substance by 1 degree C.
units: J/(grams)(degree C) |
|
|
molar heat capacity:
|
the amount of heat required to raise the temp. of 1 mole of substance by 1 degree C.
units: J/ (mol)(degree C) |
|
|
q =
|

|
|
|
Specific heat capacities of some common substances:
|

|
|
|
The equation used to quantify the relationship between the amount of heat added to a given amount of the substance and the corresponding temp. increase.
|

q is the amount of heat (J)
m is the mass of the substance (g) Cs is the specific heat capacity (J/(g)(degree C)) delta T is the temp. change (degree C) |
|
|
Pressure-Volume work:
|

occurs when the force is the result of a volume change against an external pressure.
|
|
|
101.3 J =
|
1L * atm
|
|
|
How a system exchanges energy with its surroundings via heat and pressure-volume work:
|

|
|
|
heat at constant volume:
|

|
|
|
Calorimetry:
|

the thermal energy exchanged between the reaction (the system) and the surrounding is measured by observing the change in temperature of the surrounding.
|
|
|
The temperature change related to the heat absorbed by entire calorimeter:
|

|
|

|
Total Energy Change
|
|
|
Enthalphy (H):
|
The sum of its internal energy and the product of its pressure and volume
H = E + PV |
|
|
The change in enthalpy (delta H) for any process occurring under constant pressure:
|

|
|
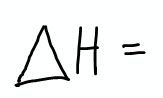
|
Represents only heat exchange
|
|
|
What does a positive delta H mean?
|
- that heat flows into the system as the reaction occurs.
- endothermic reaction. |
|
|
Endothermic reaction:
|

absorbs heat from its surroundings
uses (+) symbol feels cold to the touch |
|
|
Exothermic reaction:
|

gives off heat to its surrounding
uses (-) symbol feels warm to the touch |
|
|
If an endothermic reaction absorbs heat, then why does it feel cold to the touch?
|
an endothermic reaction feels cold to the touch because the reaction (acting here as the system) absorbs heat from the surroundings. When you touch the vessel in which the reaction occurs, you, being part of the surroundings, lose heat to the system (the reaction), which results in the feeling of cold. The heat absorbed by the reaction is not used to increase its temperature, but rather becomes potential energy stored in chemical bonds.
|
|

|
Enthalpy of reaction (heat of reaction)
- is an extensive property |
|
|
Extensive property:
|
one that depends on the amount of material.
|
|
|
Coffee-Cup Calorimeter:
|

- Measures delta H of reaction
- consists of two styrofoam coffee cups, one inserted into the other to provide insulation from the lab environment - is equipped w/ a thermometer & a stirrer. - reaction is carried out in a specifically measured quantity of solution w/in the calorimeter. heat of solution = (mass solution)(specific heat capacity of solution)(change in temp) |
|

|

|
|
|
What are the three quantitative relationships between a chemical equation and delta Hrxn?
|
1. If a chemical equation is multiplied by some factor, then delta Hrxn is also multiplied by the same factor.
2. If a chemical equation is reversed, then delta Hrxn changes sign. 3. If a chemical equation can be expressed as the sum of a series of steps, then delta Hrxn for the overall equation is the sum of the heats of reactions for each step. |
|
|
Hess's Law:
|
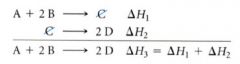
since delta Hrxn is dependent only on the initial and final states, and not on the pathway the reaction follows, the delta H obtained from summing the individual steps that lead to an overall reaction must be the same as delta H for that overall reaction.
|
|
|
Illustration of Hess's law with the energy diagram:
|

|
|
|
What are the standards for enthalpy:
|

|
|
|
Calculating the Standard Enthalpy Change for a Reaction:
|

|

