![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
77 Cards in this Set
- Front
- Back
|
Atoms and molecules determine how matter __________.
|
behaves
|
|
|
Carbon monoxide (CO)
|
a colorless gas emitted in the exhaust of vehicles
|
|
|
Atoms:
|
the submicroscopic particles that constitute the fundamental building blocks of ordinary matter
|
|
|
molecule:
|
two or more atoms joined in a specific geometric arrangement.
|
|
|
Hemoglobin:
|
the oxygen carrying protein in blood.
|
|
|
How does carbon monoxide affect us?
|
it happens to be just the right size and shape, and happens to have just the right chemical properties to fit neatly into cavities w/in hemoglobin normally reserved for oxygen.
- CO diminishes the oxygen carrying capacity of blood. - over 0.04% can kill you - small amounts will cause the heart and lungs to work harder resulting in headaches, dizziness, weakness, confusion. |
|
|
Difference between CO and CO2:
|

|
|
|
Chemistry:
|
The science that seeks to understand the behavior of matter by studying the behavior of atoms and molecules.
|
|
|
Empirical knowledge:
|
based on an observation and experiment
|
|
|
Qualitative:
|
noting/ describing how a process happens
|
|
|
Quantitative:
|
measuring or quantifying something about the process
|
|
|
Hypothesis:
|
a tentative interpretation or explanation of the obervations
|
|
|
Experiments:
|
test hypotheses, highly controlled procedures designed to generate observations.
|
|
|
Scientific law:
|
a brief statement that summarizes past observations and predicts future ones.
- describes how nature behaves - generalization about what nature does. |
|
|
Law of conservation of mass:
|
"in a chemical reaction, matter is neither created nor destroyed."
|
|
|
Scientific Theory:
|
a model for the way nature is and tries to explain not merely what nature does but why.
|
|
|
Atomic Theory:
|
- proposed by John Dalton (1766-1844)
- matter is composed of small, indestructible particles (atoms). Since these particles are merely rearranged in chemical changes (& not created/destroyed), total amount of mass remains the same. |
|
|
well-established _______ are as close to the truth as we get in science.
|
theories
|
|
|
The Scientific Method:
|
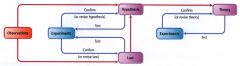
|
|
|
which of the following best explains the difference between a law and a theory?
a) a law is truth whereas a theory is mere speculation. b) a law summarizes a series of related observations, while a theory gives the underlying reasons for them. c) a theory describes what nature does; a law describes why nature does it. |
B) a law summarizes a series of related observations, while a theory gives the underlying reasons for them.
|
|
|
Matter:
|
anything that occupies space and has mass.
ex: book, desk, human, air |
|
|
Substance:
|
a specific instance of matter
ex: air, water, sand |
|
|
What are the 3 states of matter?
|

|
|
|
Solid matter:
|
- atoms or molecules packed close to each other in fixed locations.
- although the atoms and molecules in a solid vibrate, they do not move around or past each other. - has a fixed vol. and rigid shape |
|
|
solids may be _______ or _________.
|
Crystalline, amorphous
|
|
|
Crystalline:
|

in solid matter, its atoms or molecules are arranged in patterns with long-range, repeating order.
|
|
|
Amorphous:
|

in solid matter, its atoms or molecules do not have any long-range order
|
|
|
Liquid matter:
|
atoms or molecules pack about as closely as they do in a solid matter, but they are free to move relative to each other.
- fixed vol., but not a fixed shape - assume shape of their container |
|
|
gaseous matter:
|
atoms or molecules have a lot of space between them and are free to move relative to one another making gases compressible.
- always assume the shape and vol. of their container. |
|
|
How to classify matter according to its composition:
|

|
|
|
Composition:
|
the kinds and amounts of substances that composes matter
|
|
|
Pure Substance:
|
a substance composed of a single type of atom or molecule.
ex: distilled (pure) water |
|
|
Pure substances can be divided into what two types?
|
elements, compounds
|
|
|
Elements:
|
a substance that cannot be chemically broken down into simpler substances.
ex: Helium |
|
|
Compounds:
|
a substance composed of two or more elements in fixed, definite proportions.
ex: water |
|
|
Mixture:
|
a substance composed of two or more different types of atoms or molecules that can be combined in continuously variable proportions.
|
|
|
mixtures can be divided into what two types?
|
heterogeneous mixtures, homogeneous mixtures
|
|
|
Heterogeneous mixtures:
|
one in which the composition varies from one region to another.
ex: wet sand |
|
|
homogeneous mixture:
|
one with the same composition throughout.
ex: sweet tea |
|
|
Physical Change:
|
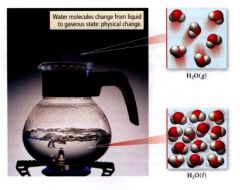
changes that alter only state or appearance but not composition.
ex: water that boils changes state from liquid to gas but composition of molecules are not changed. |
|
|
chemical Change:
|

Changes that alter the composition of matter.
- atoms rearrange ex: rusting iron. |
|
|
Chemical Change:
|

Changes that alter the composition of matter.
- atoms rearrange |
|
|
physical property:
|
a property that a substance displays without changing its composition
|
|
|
Chemical property:
|
a property that a substance displays only by changing its composition via chemical change.
|
|
|
what are some physical properties:
|
- odor
- taste - color - appearance - melting pt. - boiling pt. - density |
|
|
Chemical properties include:
|
- corrosiveness
- flammability - acidity - toxcitiy |
|
|
example of physical change and chemical change:
|
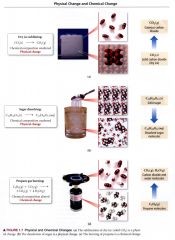
|
|

|
(a) best represents the water after vaporization. Vaporization is a physical change, so the molecules must remain the same before and after the change.
|
|
|
Energy:
|
the capacity to do work
|
|
|
Work:
|

the action of a force through a distance
|
|
|
total energy:
|
kinetic energy + potential energy
|
|
|
Kinetic energy:
|
energy associated with its motion
|
|
|
Potential energy:
|
energy associated with its position/ composition
|
|
|
thermal energy:
|
energy associated with the temperature of a object.
- actually a type of kinetic energy because it arises from the motion of the individual atoms/ molecules that make up an object. |
|
|
example of energy conversion:
|

|
|
|
Law of conservation of energy:
|
"energy is neither created nor destroyed"
the total quantity of energy does not change-- remains constant. |
|
|
"systems with high potential energy have a tendency to change in a way that lowers their potential energy"
|
summary:
- energy is always conserved in a physical or chemical change; it is neither created nor destroyed. - systems with high potential energy tend to change in a direction of lower potential energy, releasing energy into the surroundings. |
|
|
units:
|
standard quantities used to specify measurement.
|
|
|
International system of units (SI):
|
used by scientists based on metric system.
|
|
|
SI base Units:
|
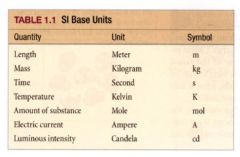
|
|
|
1kg = how many lbs?
|
2.205lbs
|
|
|
temperature scales:
|
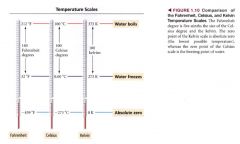
|
|
|
converting from fahrenheit to celsius:
|
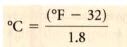
|
|
|
SI prefix Multipliers:
|

|
|
|
Derived unit:
|
combination of other units
|
|
|
volume:
|
a measure of space
|
|
|
some common units and their equivalents:
|

|
|
|
Density:
|

- a characteristic physical property of materials and differs from one substance to another
- an intensive property |
|
|
Intensive property:
|
one that is independent of the amount of the substance
|
|
|
extensive property:
|
one that depends on the amount of the substance.
|
|

|
(c) the sample expands. However, because the mass remains constant, its density decreases.
|
|
|
Significant figures rules:
|

|
|
|
Exact numbers:
|
have no uncertainty, and thus do not limit the number of significant figures in any calculation.
|
|
|
rules for calculating sig. figs:
|

|
|
|
accuracy:
|
how close the measured value is to the actual value
|
|
|
precision:
|
how close a series of measurements are to one another or how reproducible they are.
|
|
|
example of errors:
|

- inconsistency is the result of random error (error that has equal probability of being too high or too low)
- inaccuracy is the result of systematic error (error that tends toward being either too high or too low) |

