![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
205 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
define: Acid |
A substance that is able to donate a H+ ion and, hence, increases the concentration when it dissolves in water. |
|
|
|
define: alcohol |
an organic compound obtained by substituting a hydroxyl group (-OH) for a hydrogen on a hydrocarbon |
-OH |
|
|
Avogadro's Number |
the number of 12C atoms in exactly 12g of 12C =6.022x10^23 mol^-1 |
|
|
|
define: base |
A substance that is an H+ acceptor; a base produces an excess of OH- ions when it dissolves in water |
OH- |
|
|
define: Capillary Action |
the process by which a liquid rises in a tube because of a combination of adhesion to the walls of the tube and cohesion between liquid particles |
|
|
|
define: Cholesteric Liquid Crystalline Phase |
a liquid crystal formed from flat, disc-shaped molecules that align through a stacking of the molecular discs |
|
|
|
define: colloids (colloidal dispersions) |
mistures containing particles larger than normal solutes but small enough to remain suspended in the dispersing medium |
|
|
|
define: colligative property |
a property of a solvent that depends on the total concentration of solute particles present |
|
|
|
Types of Colligative Property |
1. Vapor-Pressure Depression 2. Freezing-Point Depression 3. Boiling-Point Elevation 4. Osmotic Pressure |
4 |
|
|
define: Critical Pressure |
the pressure at which a gas at its critical temperature is converted to a liquid state |
|
|
|
define: Critical Temperature |
the highest temperature at which it is possible to convert the gaseous form of a substance to a liquid. The critical temperature increases with an increase in the magnitude of intermolecular forces |
|
|
|
define: Dipole-Dipole Force |
a force that becomes significant when polar molecules come in close contact with one another. The force is attractive when the positive end of one polar molecule approaches the negative end of another |
|
|
|
define: Dispersion Forces |
intermolecular forces resulting from attractions between induced dipoles. Also called London dispersion forces |
|
|
|
define: Dynamic Equilibrium |
a state of balance in which opposing processes occur at the same rate |
|
|
|
define: Henry's Law |
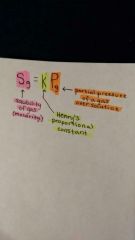
a law stating that the concentration of a gas in a solution. |
|
|
|
define: ideal solution |
a solution that obeys Raoult's law |
|
|
|
define: immiscible liquids |
liquids that do not dissolve in one another to a significant extent |
|
|
|
define: Intermolecular Forces |
the short-range attractive forces operating between the particles that make up the units of a liquid or solid substance. These same forces also cause gases to liquefy or solidify at low temperatures and high pressures |
|
|
|
define: Ion-Dipole Force |
the force that exists between an ion and a neutral polar molecule that possesses a permanent dipole moment |
|
|
|
define: Liquid Crystal |
a substance that exhibits one or more partially ordered liquid phases above the melting point of the solid form. By contrast, in nonliquid crystalline substances the liquid phase that forms upon melting is completely unordered |
|
|
|
define: Mass Percentage |

the number of grams of solute in each 100g of solution |
|
|
|
define: Miscible Liquids |
liquids that mix in all proportions |
|
|
|
define: molality |
the concentration of a solution expressed as moles of solute per kilogram of solvent; abbreviated m |
|
|
|
define: Nematic Liquid Crystalline Phase |
a liquid crystal in which the molecules are aligned in the same general direction, along their long axes, but in which the ends of the molecules are not aligned |
|
|
|
define: Normal Boiling Point |
the boiling point at 1 atm pressure |
|
|
|
define: Normal Melting Point |
the melting point at 1 atm pressure |
|
|
|
define: Osmosis |
the net movement of solvent through a semipermeable membrane toward the solution with greater solute concentration |
|
|
|
define: Osmotic Pressure |
the pressure that must be applied to a solution to stop osmosis from pure solvent into the solution |
|
|
|
define: Phase Change |
the conversion of a substance from one state of matter to another |
|
|
|
define: Vaporization |
liquid to gas |
|
|
|
define: fusion |
solid to liquid |
|
|
|
define: freezing |
liquid to solid |
|
|
|
define: Condensation |
gas to liquid |
|
|
|
define: Sublimation |
solid to gas |
|
|
|
define: Deposition |
gas to solid |
|
|
|
define: Polarizability |
the ease with which the electron cloud of an atom or a molecule is distorted by an outside influence, thereby inducing a dipole moment |
|
|
|
define: Raoult's Law |

a law stating that the partial pressure of a solvent over a solution, Psolution, is given by the vapor pressure of a pure solvent, Psolvent, times the mole fraction of a solvent in the solution |
|
|
|
define: Saturated Solution |
a solution in which undissolved solute and dissolved solute are in equilibrium |
|
|
|
define: Solubility |
the amount of a substance that dissolves in a given quantity of solvent at a given temperature to form a saturated solution |
|
|
|
define: Solvation |
the clustering of solvent molecules around a solute particle |
|
|
|
define: Supersaturated Solution |
a solution containing more solute than an equivalent saturated solution |
|
|
|
define: Surface Tension |
the intermolecular, cohesive attraction that causes a liquid to minimize its surface area |
|
|
|
define: Triple Point |
the temperature at which solid, liquid, and gas phases coexist in equilibrium |
|
|
|
define: Tyndall Effect |
the scattering of a beam of visible light by the particles in a colloidal dispersion |
|
|
|
define: Vapor Pressure |
the pressure exerted by a vapor in equilibrium with its liquid or solid phase |
|
|
|
define: Viscosity |
a measure of the resistance of fluids to flow |
|
|
|
define: Volatile |
tending to evaporate readily |
|
|
|
We can change a substance from one state to another by heating or cooling, which changes the __________________ of the particles. |
average kinetic energy |
|
|
|
define: Solid |
matter that has both a definite shape and definite volume |
|
|
|
define: Ion-Dipole |
permanent dipole moment between an ion and neutral polar molecule |
|
|
|
As the temperature of a gas decreases, the average kinetic energy of its particles decreases, allowing the attractions between the particles to first draw the particles close together, forming a liquid, and then to virtually lock them in place, forming a solid. |
Kinetic energy decrease causes particles to get close enough together to experience intermolecular forces. |
|
|
|
______________ forces are generally much weaker than ______________ forces. |
Intermolecular / Intramolecular |
Inter & Intra |
|
|
As the strengths of the intermolecular forces increase, the boiling point and melting point _____________. |
increases. |
increase or decrease? |
|
|
Types of Intermolecular Forces |
1. Dispersion Forces 2. Dipole-Dipole Forces 3. Hydrogen Bonding 4. Ion-Dipole Forces |
|
|
|
Dispersion Forces |
London recognized that the motion of electrons in an atom or molecule can create an instantaneous, or momentary, dipole moment. |
|
|
|
Dispersion forces are significant only when molecules are _____________. |
very close together |
|
|
|
define: Polarizability |
the ease with which the charge distribution is distorted |
|
|
|
Relation between polarizability and dispersion forces |
More polarizable molecules have larger dispersion forces. |
|
|
|
Dispersion forces tend to ___________ in strength with increasing molecular weight. |
increase |
increase or decrease |
|
|
Dipole-Dipole Forces |
The presence of a permanent dipole moment in polar molecules gives rise to dipole-dipole forces. These forces originate from electrostatic attractions between the partially positive end of one molecule and the partially negative end of a neighboring molecule. |
|
|
|
The presence of a permanent dipole moment in polar molecules gives rise to ___________ forces. These forces originate from electrostatic attractions between the partially positive end of one molecule and the partially negative end of a neighboring molecule. |
dipole-dipole |
|
|
|
These forces originate from electrostatic attractions between the partially positive end of one molecule and the partially negative end of a neighboring molecule. |
dipole-dipole forces |
|
|
|
For molecules of approximately ______ mass and size, the strenght of intermolecular attractions increases with increasing polarity. |
equal |
|
|
|
For molecules of approximately equal mass and size, the strength of intermolecular attractions ______ with increasing polarity. |
increasing |
|
|
|
A ___________ is an attraction between a hydrogen atom attached to ahighly electronegative atom (usually F, O, and N) and a nearby small electronegative atom in another molecule or chemical group |
hydrogen bond |
|
|
|
define: Hydrogen Bond |
an attraction between a hydrogen atom attached to a highly electronegative atom (usually F, O, and N) and anearby small electronegative atom in another molecule or chemical group |
|
|
|
define: Ion-Dipole Forces |
Cations are attracted ot the negative end of a dipole, and anions are attracted to the positive end |
|
|
|
An ________ exists between an ion and a polar molecule |
ion-dipole force |
|
|
|
_________ forces are found in all substances. |
dispersion |
|
|
|
When comparing the relative strengths of intermolecular attrations, consider these generalizations: |
1. When the molecules of two substances have comparable molecular weights and shapes, dispersion forces are approximately equal in the two substances. 2. When the molecules of two substances differ widely in molecular weights, and there is no hydrogen bonding, dispersion forces tend to determine which substance has the stronger intermolecular attractions. |
|
|
|
When the molecules of two substances have comparable molecular weights and shapes, dispersion forces are _________ in the two substances. |
appoximately equal |
|
|
|
When the molecules of two substances have _________ molecular weights and shapes, dispersion forces are approximately equal in the two substances. |
comparable |
|
|
|
When the molecules of two substances differ widely in molecular weights, and there is no hydrogen bonding, _________ tend to determine which substance has the stronger intermolecular attractions. |
dispersion forces |
|
|
|
Ions present? NO Polar Molecules present? NO |
Dispersion forces only |
|
|
|
Ions present? NO Polar molecules present? YES Hydrogen bonding? NO |
dipole-dipole forces |
|
|
|
Ions Present? NO Polar Molecules Present? YES Hydrogen Bonding? YES |
Hydrogen bonding |
|
|
|
Ions Present? YES Polar Molecules Present? NO |
Ionic Bonding |
|
|
|
Ions Present? YES Polar Molecules Present? YES |
Ion-Dipole forces |
|
|
|
When the molecules of two substances have comparable molecular weights and shapes, the differences in the magnitudes of the intermolecular forces are due to differences in the strengths of _________ attractions. |
dipole-dipole |
|
|
|
define: viscosity |
the resistance of a liquid to flow |
|
|
|
The greater a liquid's viscosity, the more _______ it flows. |
slowly |
|
|
|
viscosity ______ with increasing temperature |
decreases |
|
|
|
viscosity decreases with _____ temperature |
increasing |
|
|
|
define: Surface Tension |
energy required to increase the surface area of a liquid by a unit amount |
|
|
|
A measure of the net inward force that must be overcome to expand the surface area of a liquid is given by its |
surface tension. |
|
|
|
Intermolecular forces that bind similar molecules to one another, such as the hydrogen bonding in water, are called ________. |
Cohesive Forces |
|
|
|
Intermolecular forces that bind a substance to a surface are called __________. |
adhesive forces |
|
|
|
The rise of liquids up very narrow tubes is called ________. |
Capillary Action |
|
|
|
The __________ forces between the liquid and the walls of the tube tend to increase the surface area of the liquid. |
adhesive |
|
|
|
Vapor pressure __________ with increasing temperature until it equals the external pressure above the liquid, typically atmospheric pressure. |
increases |
|
|
|
Vapor pressure increases with ______ temperature until it equals the external pressure above the liquid, typically atmospheric pressure. |
increasing |
|
|
|
Vapor pressure increases with increasing temperature until it equals the external pressure above the liquid, typically ________. |
atmospheric pressure |
|
|
|
Heat of deposition for a given substance is exothermic to the same degree that the __________ is endothermic. |
heat of sublimation |
|
|
|
___________ for a given substance is exothermic to the same degree that the heat of sublimation is endothermic. |
Heat of deposition |
|
|
|
Heat of Deposition: endothermic or exothermic? |
exothermic |
|
|
|
Heat of sublimation: endothermic or exothermic |
endothermic |
|
|
|
The _____________ is exothermic to the same degree that the heat of fusion is endothermic. |
Heat of freezing |
|
|
|
The heat of freezing is exothermic to the same degree that the _________ is endothermic. |
heat of fusion |
|
|
|
The heat of Freezing: endothermic or exothermic |
exothermic |
|
|
|
Heat of Fusion: endothermic or exothermic |
endothermic |
|
|
|
define: Heating Curve |
a graph of temperature versus amount of heat added |
|
|
|
A graph of temperature versus amount of heat added is called a ___________. |
Heating Curve |
|
|
|
The highest temperature at which a distinct liquid phase can form is called the _____________. |
Critical Temperature |
|
|
|
define: critical temperature |
the highest temperature at which a distinct liquid phase can form |
|
|
|
The _____________ is the pressure required to bring about liquefaction at this critical temperature. |
critical pressure |
|
|
|
define: Critical Pressure |
pressure required to bring about liquefaction at critical the critical temperature |
|
|
|
The greater the intermolecular forces, the ________ the critical temperature of a substance. |
higher |
|
|
|
Nonpolar, Low Molecular Weight molecules have ________ intermolecular attractions that substances that are polar or have higher molecular weight. |
weaker |
|
|
|
Nonpolar, low-molecular-weight substances have ________ critical temperatures and pressures than substances that are polar or of higher molecular wight. |
lower |
|
|
|
It is useless to try to liquefy a gas by applying pressure if the gas is above its _______________. |
critical temperature |
|
|
|
define: Supercritical Fluid |
state in which the liquid and gas phases are indistinguishable from each other |
|
|
|
When the temperature exceeds the critical temperature and the pressure exceeds the critical pressure, the substances reaches a state called __________. |
supercritical fluid |
|
|
|
define: Dynamic Equilibrium |
the condition in which two opposing processes occur simultaneously at equal rates |
|
|
|
The condition in which two opposing processes occur simultaneously at equal rates is called _______. |
Dynamic Equilibrium |
|
|
|
define: Vapor Pressure |
the pressure exerted by its vapor when the liquid and vapor are in dynamic equilibrium |
|
|
|
The ____________ of a liquid is the pressure exerted by its vapor when the liquid and vapor are in dynamic equilibrium. |
Vapor Pressure |
|
|
|
The vapor pressure of a liquid is the pressure exerted by its vapor when the liquid and vapor are in ____________. |
Dynamic Equilibrium |
|
|
|
The __________ of a liquid is the temperature at which its vapor pressure equals the external pressure, acting on the liquid surface. |
boiling point |
|
|
|
The boiling point of a liquid is the temperature at which its vapor pressure equals the ____________, acting on the liquid surface. |
external pressure |
|
|
|
The boiling point of a liquid at 1 atm pressure is called its ____________. |
Normal Boiling Point |
|
|
|
define: Normal Boiling Point |
the boiling point of a liquid at 1 atm pressure |
|
|
|
The temperature at which solid and liquid phases coexist at equilibrium is the __________ of the solid. |
Melting point |
|
|
|
The temperature at which solid and liquid phases coexist at equilibrium is the __________ of the liquid. |
Freezing point |
|
|
|
A ___________ allows us to predict which phase of a substance is present at any given temperature and pressure. |
phase diagram |
|
|
|
The ________ corresponds to the critical temperature and critical pressure of the substance. |
critical point |
|
|
|
At temperatures and pressures beyond the critical point, the liquid and gas phases are indistinguishable from each other, and the substance is a ____________. |
supercritical fluid |
|
|
|
The _____________ separates the solid phase from the gas phase and represents the change in the vapor pressure of the solid as it sublimes at different temperatures. |
sublimation curve |
|
|
|
define: Sublimation Curve |
line on a phase diagram that separates the solid phase from the gas phase and represents the change in the vapor pressure of the solid as it sublimes at different temperatures |
|
|
|
The _________ separates the solid phase from the liquid phase and represents the change in melting point of the solid with increasing pressure. |
melting curve |
|
|
|
define: melting curve |
line on a phase diagram that separates the solid phase from the liquid phase and represents the change in melting point of the solid with increasing pressure |
|
|
|
The melting point at 1 atm pressure is the ________. |
Normal Melting Point |
|
|
|
define: Normal Melting Point |
The melting point at 1 atm pressure |
|
|
|
define: Liquid Crystal |
viscous, milky state that some substances exhibit between the liquid and solid states |
|
|
|
Types of Liquid Crystals |
1. Nematic 2. Smectic A 3. Smectic C 4. Cholesteric |
4 |
|
|
define: Nematic Liquid Crystalline Phase |
Crystalline Phase in which the molecules are aligned so that their long axes tend to point in the same direction but the ends are not aligned with one another. |
|
|
|
Crystalline Phase in which molecules are aligned so that their long axes tend to point in the same direction but the ends are not aligned with one another. |
Nematic |
|
|
|
define: Smectic C Liquid Crystals |
Crystalline Phase in which the molecules maintain the long-axis alignment seen in nematic crystals, but in addition they pack into layers. |
|
|
|
Crystalline Phase in which the molecules maintain the long-axis alignment seen in nematic crystals, but in addition they pack into layers. |
Smectic A and C Liquid Crystals |
|
|
|
In _______ liquid crystals, the intermolecular forces limit the ability of the molecules to slide past one another. |
smectic A and C |
|
|
|
define: Cholesteric Liquid Crystal |
Crystalline Phase in which the molecules are arranged in layers, with their long axes parallel to the other molecules within the same layer |
|
|
|
In a ________ crystal, the molecules are arranged in layers, with their long axes parallel to the other molecules within the same layer. |
cholesteric |
|
|
|
When the molecules mix and become more randomly distributed, there is an increase in __________. |
entropy |
|
|
|
3 types of intermolecular interactions that are involved in solution formation |
1. solute-solute 2. solvent-solvent 3. solvent-solute |
|
|
|
_______ interactions between solute particles must be overcome to disperse the solute particles through the solvent. |
solute-solute |
|
|
|
_________ interactions between solvent particles must be overcome to make room for the solute particles in the solvent. |
Solvent-Solvent |
|
|
|
___________ interactions between the solvent and solute particles occur as the particles mix |
solvent-solute |
|
|
|
Solutions form when the magnitudes of the solvent-solute interactions are ___________ the solute-solute and solvent-solvent interactions. |
either greater than or equal to |
|
|
|
define: solvation |
interactions between solute and solvent molecules |
|
|
|
Interactions between solute and solvent molecules are known as _______. |
solvation |
|
|
|
When the solvent is water, the interactions are referred to as ____________. |
hydration |
|
|
|
Solution processes are typically accompanied by changes in _______. |
enthalpy |
|
|
|
Enthalpy Change is the sum of three steps |
1. Enthalpy of Solute 2. enthalpy of solvent 3. enthalpy of mix |
|
|
|
Separation of the solute particles from one another is endothermic or exothermic? |
endothermic |
|
|
|
Seperation of solvent molecules to accommodate the solute is endothermic or exothermic? |
endothermic |
|
|
|
In attractive interactions between solute particles and solvent particles is endothermic or exothermic? |
exothermic |
|
|
|
Compounds that have a defined number of water molecules in the crystal lattice are known as _______. |
hydrates |
|
|
|
define: hydrate |
compounds with a defined number of water molecules in the crystal lattice |
|
|
|
the opposite of the solution process is the ______ |
crystallization |
|
|
|
A solution that is in equilibrium with undissolved solute is _____. |
saturated |
|
|
|
In a ________ solution, additional solute will not dissolve if added to a saturated solution. |
saturated |
|
|
|
The amount of solute needed to forma saturated solution in a given quatity of solvent is known as the _______. |
solubility |
|
|
|
define: solubility |
maximum amount of the solute that can dissolve in a given amount of the solvent at a specified temperature, assuming that excess solute is present |
|
|
|
It is possible to form solutions that contain a greater amount of solute than needed to form a saturated solution. This is called ______. |
supersaturated |
|
|
|
define: supersaturated |
A solution containing a greater amount of solution than needed to form a saturated solution |
|
|
|
In general, when other factors are comparable, the _______ the attractions between solute and solvent molecules, the greater the solubility of the solute in that solvent. |
stronger |
|
|
|
In general, when other factors are comparable, the stronger the attractions between solute and solvent molecules, the _____ the solubility of the solute in that solvent. |
greater |
|
|
|
Liquids that mix in all proportions are _______. |
miscible |
|
|
|
Liquids that do not dissolve in one another are ______. |
immiscible |
|
|
|
Organic compounds tha contain the polar OH group are called _____. |
alcohols |
|
|
|
The solubility of alcohol in water decreases as the number of carbon atoms in an alcohol ______. |
increases |
|
|
|
The solubility of alcohols in water _______ as the number of carbon atoms in an alcohol increases. |
decreases |
|
|
|
The solubility of alcohols in a nonpolar solvent ______ as the nonpolar hydrocarbon chain lengthens. |
increases |
|
|
|
One way to enhance the solubility of a substance in water is to increase the number of ________ the substance contains. |
Polar groups |
|
|
|
The solubility of a gas in any solvent is _____ as the partial pressure of the gas above the solvent increases. |
increased |
|
|
|
The solubility of a gas in any solvent is increased as the partial pressure of the gas above the solvent _________. |
increases |
|
|
|
The solubility of a gas in a liquid solvent ______ in direct proportion to the partial pressure of the gas above the solution. |
increases |
|
|
|
The solubility of a gas in a liquid solvent increases in direct proportion to the _____ of the gas above the solution. |
partial pressure |
|
|
|
A solution with a relatively small concentration of solute is said to be _______. |
dilute |
|
|
|
define: dilute solution |
a solution with a relatively small concentration of solute |
|
|
|
A solution with a large concentration is said to be _____________ |
concentrated |
|
|
|
Mass Percentage |

|
|
|
|
Mole Fraction |

|
|
|
|
Malarity |
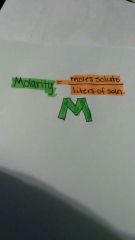
|
|
|
|
molality |

|
|
|
|
Molarity depends on the _______ of solution. |
volume |
|
|
|
Molality depends on the _______ of solvent. |
mass |
|
|
|
To convert between molality and molarity, the _______ will be needed. |
density |
|
|
|
Physical properties of solutions that depend on the quantity (concentration) but not on the kind or identity of the solute particles are called __________. |
Colligative Properties |
|
|
|
The _______ is the pressure exerted by the vapor when it is at equilibrium with the liquid. |
vapor pressure |
|
|
|
A substance that has no measurable vapor pressure is __________. |
nonvolatile |
|
|
|
define: nonvolatile |
a substance that has no measurable vapor pressure |
|
|
|
A substance that exhibits a vapor pressure is ______. |
volatile |
|
|
|
define: volatile |
a substance that exhibits a vapor pressure |
|
|
|
Raoult's Law |
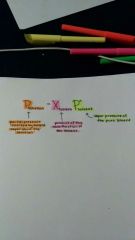
|
|
|
|
In boiling-point elevation, the vapor-pressure curve of the solution is shifted _________ relative to the vapor-pressure curve of the pure solvent. |
downward |
|
|
|
The boiling point of a solution is _____ than that of the pure solvent. |
higher |
|
|
|
In Boiling-Point Elevation, the increase in the boiling point of a solution, relative to the pure solvent, depends on the _______ of the solute. |
molality |
|
|
|
Freezing Point Depression Equation |

|
|
|
|
Boiling Point Elevation Equation |

|
|
|
|
define: osmosis |
the net movement of solvent is always toward the solution with the lower solvent (higher solute) concentration |
|
|
|
Osmotic Pressure Equation |

|
|
|
|
In one solution is of lower osmotic pressure, it is _______ with respect to the more concentrated solution. |
hypotonic |
|
|
|
define: hypotonic |
lower osmotic pressure with respect to the more concentrated solution |
|
|
|
the more with concentrated solution is _________ with respect to the dilute solution. |
hypertonic |
|
|
|
define: hypertonic |
more concentrated with respect to the dilute solution |
|

