![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
80 Cards in this Set
- Front
- Back
|
Kinetic |
Came from greek kinesis which means movement |
|
|
Kinetic theory (Kinetic Molecular Theory in Gases) |
Always associated in describing gas as particles in random motion |
|
|
Kinetic molecular theory in liquids and solids |
Explains attraction which forms sllid and liquid phases |
|
|
Intermolecular force |
Attractive force between molecules |
|
|
Intermolecular and intramolecular forces |
2 forces involved in liquid and solid molecules |
|
|
Intramolecular forces |
Holding atoms together (to molecules) |
|
|
Intermolecular forces |
Generally much weaker than intramolecular forces (bonds) |
|
|
Ion-dipole forces |
Bond between ion and polar molecule |
|
|
Dipole-dipole forces |
Attractive forces between polar molecules -between neighboring molecules with permanent dipoles |
|
|
Hydrogen bond |
Special dipole dipole interaction between hydrogen atom in polar N-H, O-H, or F-H bond and on electronegativity O, N, or F atom |
|
|
Dispersion forces |
Induced-dipole / van der waals forces / London forces |
|
|
Polarity |
Results in uneven partial distribution of a molecule between various atom groups |
|
|
Dipole moment |
Direceion of the polar bond of a molecule |
|
|
Polar molecules |
Asymmetric, en diff 0.5-1.7, net dipole moment, diatomic molecules of different elements, molecules with O, N, or OH at one end |
|
|
Non polar molecules |
Symmetric, en diff 0-0.4, dipole moments are cancelled out, molecules of elements sharing electrons equally |
|
|
Polarizability |
Ease with which electron distribution can be distorted |
|
|
Vapor pressuree |
In a sealed container, some liquid evaporates to establish a pressure in the vapor phase, Vapor pressure: partial pressure of the vapor over the liquid measured at equilibrium and at some temperature. • Dynamic equilibrium |
|
|
Clausius-Clapeyron equation |
shows how the vapor pressure and temperature are related. |
|
|
Boiling point |
the temperature at which the vapor pressure of a liquid is equal to the pressure of the external atmosphere. |
|
|
Normal boiling point |
The temperature at which the vapor pressure of a liquid is equal to atmospheric pressure (1 atm). |
|
|
Heat of vaporization |
heat needed for the vaporization of a liquid. |
|
|
Heat of fusion |
heat needed for the melting of a liquid. |
|
|
Surface tension |
is the amount of energy required to stretch or increase the surface of a liquid by a unit area, it is a force that causes the molecules on the surface of the liquid to be pushed together (contract) and form a layer. |
|
|
Cohesion |
is the intermolecular attraction between like molecules, responsible for surface tension |
|
|
Adhesion |
is the intermolecular attraction between unlike molecules |
|
|
Viscosity |
is a measure of a fluid’s resistance to flow. |
|
|
Fluidity |
A measure of how molecules move/flow “freely” with one another. |
|
|
Ionic solid |
ionic bonds hold the solids in a regular three dimensional arrangement |
|
|
Molecular solid |
solids like ice that are held together by intermolecular forces. |
|
|
Covalent network |
a solid consists of atoms held together in large networks or chains by covalent networks. |
|
|
Metallic |
similar to covalent network except with metals. Provides high conductivity. |
|
|
Metal crystals |
made up of atoms in regular arrays |
|
|
Unit cell |
the smallest of repeating array of atoms |
|
|
Crystalline solid |
a well defined arrangement of atoms; this arrangement is often seen on a macroscopic level. |
|
|
Amorphous solid |
atoms are randomly arranged. No order exists in the solid. |
|
|
3 types of cubic cells |
Simple cubic, body centered cubic, face centered cubic |
|
|
Simple cubic |
All sides equal, all angles 90° |
|
|
Tetragonal |
2 sides (a and b) equal, all angles 90° |
|
|
Orthorhombic |
All sides unequal, all angles 90° |
|
|
Rhombohedral |
All sides equal, 2 angles 90° |
|
|
Monoclinic |
All sides unequal, 2 angles 90° |
|
|
Triclinic |
All sides unequal, no angles 90° |
|
|
Hexagonal |
2 sides are equal, 2 angles 90° and 1 120° |
|
|
Melting |
change of a solid to a liquid. |
|
|
Freezing |
Liquid to solid |
|
|
Vaporization |
Liquid to gas |
|
|
Sublimation |
Solid to gas |
|
|
Condensation |
Gas to liquid |
|
|
Deposition |
Gas to solid |
|
|
Phase Diagram |
Graph of pressure-temperature relationship; describes when 1,2,3 or more phases are present and/or in equilibrium with each other, lines indicate equilibrium state of two phases. |
|
|
Critical temperature |
Temperature where substance must always be gas, no matter what the pressure is. |
|
|
Triple point |
Temperature and pressure where all three phases co-exist in equilibrium |
|
|
Critical point |
point where system is at its critical pressure and temperature |
|
|
Critical pressure |
vapor pressure at critical temperature |
|
|
Phase diagram |
summarizes the conditions at which a substance exists as a solid, liquid, or gas |
|
|
Solution |
•a mixture of two or more substances that is identical throughout |
|
|
Solute |
is the substance being dissolved |
|
|
Solvent |
is the medium in which the solute is dissolved |
|
|
Solubility |
Refers to maximum amount of solute that can be dissolved under specific temperature and pressure conditions |
|
|
Insoluble |
A substance that cannot dissolve or only to a very limited extent is called |
|
|
Miscibility |
Ability of a liquid to solute and dissolve to another |
|
|
Factors affecting solubility |
A. Nature of solute and solvent B. Effect of Temperature C. Effect of Pressure D. Surface Area E. Stirring |
|
|
Miscible; immiscible |
Able to mix; unable to mix (liquid) |
|
|
final observable phase |
Solutions can be classified based on the _ -gaseous solution, liquid solution or solid solution |
|
|
Amount of solute present |
Solutions can be classified based on the _ -dilute solution, concentrated solution |
|
|
amount of solute dissolved in the given solvent |
Solutions can also be classified in relation to the __ -unsaturated solution, saturated solution, supersaturated solution |
|
|
Types of solutions |
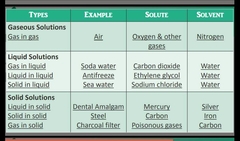
|
|
|
Concentration of solutions |
Solutions can be described qualitatively or quantitatively based on the amount of solute relative to a given amount of solvent. |
|
|
Dilute solution |
contains a relatively small amount of solute |
|
|
Concentrated |
solution contains a relatively large amount of solute. |
|
|
Unsaturated solution |
contains less solute than the solvent has the capacity to dissolve at a specific temperature. |
|
|
Saturated solution |
contains the maximum amount of a solute that will dissolve in a given solvent at a specific temperature. |
|
|
Supresaturated solution |
contains more solute than is present in a saturated solution at a specific temperature. |
|
|
Ways of expressing the concentration of solutions |
1. Parts per Million & Parts per Billion 2. Percent by Mass & Percent by Volume 3. Mole Fraction 4. Molarity 5. Molality |
|
|
Parts per Million (ppm) & Parts per Billion (ppb) used |
used when the concentration of solute present is very low |
|
|
Ppm and ppb |
unit for expressing very dilute concentrations. It is commonly used to express the concentration of pollutants in air or in water |
|
|
Mass/volume percent |
One way of representing the concentration of an element in a compound or a component in a mixture. It gives the mass of solute dissolved in a volume of solution, in a percent |
|
|
Mole Fraction |
is the ratio of the number of moles of one component to the total number of moles in a solution represented by capital X |
|
|
Molarity |
is the ratio of the number of moles per liter of solution |
|
|
Molality |
defined as the number of moles of solute per kilogram of solvent |

