![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
Review of Isomerism. Explain.
|
Two different types of isomers exist:
Constitutional isomers (also called structural isomers): Different connectivity of atoms. Stereoisomers: Same connectivity of atoms but different configuration. |
|
|
Constitutional isomers can arise from three types of connectivity differences: What are they?
|
(1) Different carbon skeletons, (2) Different placements of functional groups on the same skeleton, & (3) Different functional groups together with different skeletons.
|
|
|
Explain Different carbon skeletons?
|
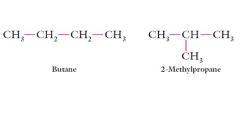
|
|
|
Explain Different placements of functional groups on the same skeleton?
|
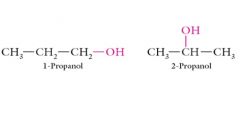
|
|
|
Different functional groups together with different skeletons
|
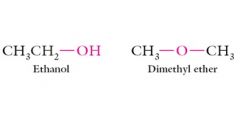
|
|
|
What are some Stereoisomer properties?
|
Stereoisomers differ in configuration, or orientation in space independent of changes the occur by bond rotation about single bonds.
|
|
|
Enantiomers: stereoisomers that are non-superimposable mirror images of each other.
|
|
|
|
What are Diastereomers?
|
Diastereomers: stereoisomers that are not enantiomers (not mirror images of each other).
|
|
|
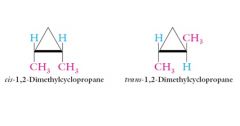
Geometrical stereoisomers or cis-trans stereoisomers: Two carbons of a double bond or a ring contain two different substituents.
|
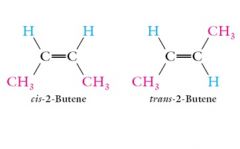
|
|
|
Enantiomers are pairs of compounds that meet the following conditions:
The molecules of the two compounds are mirror images of each other. (A mirror image of a right hand looks like a left hand.) The molecules of the two compounds are nonsuperimposable on each other. (A right hand cannot be superimposed on a left had regardless of the orientation of either.) |

|
|
|
A molecule that is not superimposable on its mirror images is said to be chiral or to have chirality. Molecules having superimposable mirror images are said to be achiral.
|
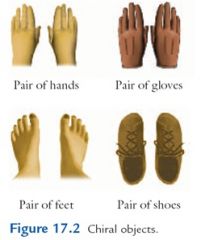
|
|
|
2-Butanol is an example of a chiral molecule. All of the atoms of the molecule and its mirror image superimpose except the –H and –CH3 groups. Show me?
|
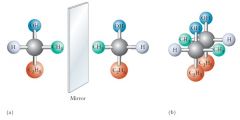
|
|
|
Two alternate representations of the 2-butanol enantiomers are what representations?
|
The wedge-bond and Fischer-projection representations
|
|
|
Wedge-bond are Solid, horizontal wedges extend in front of the plane of the paper.
Dashed, vertical wedges extend behind the plane of the paper. The wider ends of either type of wedge are nearer the viewer. Show me? |
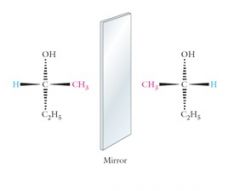
|
|
|
Fischer-projection is Horizontal bonds extend in front of the plane of the paper.
Vertical bonds extend behind the plane of the paper. Show me? |
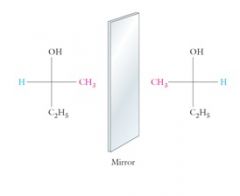
|
|
|
Consider the molecule, 2-methyl-2-butanol. Because its mirror images are superimposable, it is not a chiral compound. Show me?
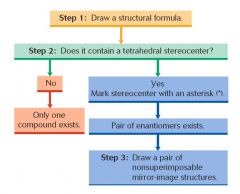
A simpler test than comparing mirror images can be used to determine chirality: Unless a compound contains a tetrahedral stereocenter (an acyclic carbon atom having four different substituents), its mirror image will be superimposable and it is not a chiral compound. |
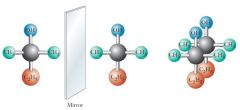
|
|
|
Enantiomers or Achiral steps. show me?
|
|
|
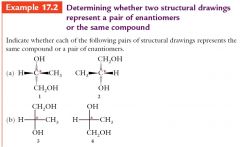
|
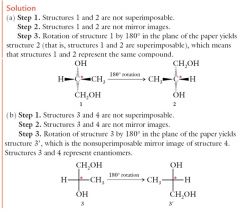
|
|
|
Explain Nomenclature of Enantiomers?
|
Each enantiomer of a pair of enantiomers is specified by adding a prefix to the name of the compound. This is done once the configuration of the enantiomer has been determined by a technique called x-ray crystallography.
Two systems of nomenclature are used for enantiomers: D- and L-: the original system. (R)- and (S)-: a more recent system. The D/L system is simpler than the R/S system and is used extensively in biochemistry and biology to name carbohydrates, amino acids, and other important biochemicals. The D/L system will be used in this course. |
|
|
Explain Properties of Enantiomers
|
Chiral compounds exhibit a property called optical activity and are said to be optically active.
Achiral molecules are optically inactive. Optical activity is the only property that distinguishes one enantiomer from the other. All other properties (MP, BP, solubility, etc.) are the same. Optical activity is the ability of a compound to rotate the plane of plane-polarized light. |
|
|
The rotation of plane-polarized light is measured by an instrument called a polarimeter.
(1) Plane-polarized light is unaffected by a solution of achiral molecules. (2) The polarization plane of plane-polarized light is rotated by a solution of an enantiomer of a chiral compound. |
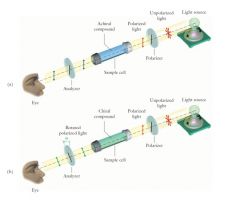
|
|
|
Explain the two enantiomers of a pair of enantiomers rotate the plane of plane-polarized light the same number of degrees but in opposite directions.
|
The two enantiomers of a pair of enantiomers rotate the plane of plane-polarized light the same number of degrees but in opposite directions.
Rotation in the clockwise direction is called dextrorotatory (+) and in the anticlockwise direction, levorotary (-). D- and L- are not directly related to (+) and (-). When both the configuration and the optical rotation are known for a compound, both are indicated in the name: D-(+)-glyceraldehyde D-(-)-fructose |
|
|
Rotation in the clockwise direction is called what?
|
Rotation in the clockwise direction is called dextrorotatory (+)
|
|
|
Rotation in the anticlockwise direction is?
|
Rotation in the anticlockwise direction is levorotary (-).
|
|
|
An equimolar mixture of two enantiomers does not exhibit optical activity.
The rotation of light in one direction by one enantiomer is exactly balanced by the rotation of light in the other direction by the other enantiomer. What is such a mixture called? |
An equimolar mixture of two enantiomers does not exhibit optical activity.
The rotation of light in one direction by one enantiomer is exactly balanced by the rotation of light in the other direction by the other enantiomer. Such a mixture of enantiomers is called a racemic mixture or racemate. |
|
|
Chemical Properties: Chiral Recognition
The reactivity of an enantiomer of a chiral compound depends upon the nature of the other reactants or the enzyme (biological catalyst) or both |
|
|
|
An analogy to the behavior of reacting chiral and achiral molecules is the relationship of what type of examples?
|
An analogy to the behavior of reacting chiral and achiral molecules is the relationship of feet (chiral), socks (achiral), and shoes (chiral):
Either achiral sock will fit onto either chiral foot, or into either chiral shoe. Only the left shoe will fit the left foot, and the right shoe the right foot, however. |
|
|
Most reactions in living cells are catalyzed by protein molecules called enzymes.
Enzymes are large molecules containing a surface site, called the active site, where the substrate or reactant binds. For most enzymes this site is chiral. Chiral active sites are designed to interact strongly with one of the two possible substrate enantiomers, just as a left shoe interacts strongly with a left foot, and more or less excludes a right foot. This phenomenon is called what? |
This phenomenon is called chiral recognition or chiral discrimination.
|
|
|
Other molecular properties are also used by enzymes to discriminate between substrate molecules: geometrical isomerism, size, shape, polarity, and charge.
Discrimination on these bases is called what? |
Other molecular properties are also used by enzymes to discriminate between substrate molecules: geometrical isomerism, size, shape, polarity, and charge.
Discrimination on these bases is called the complementarity principle. |
|
|
Show me some properties of enantiomers?
|
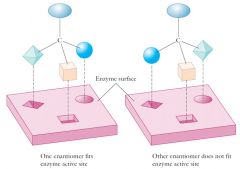
|
|
|
The enzymes used to catalyze most biological processes have evolved to utilize only one enantiomer in each class of biological compounds.
What are those compounds? |
The enzymes used to catalyze most biological processes have evolved to utilize only one enantiomer in each class of biological compounds.
D-carbohydrates L-proteins Biological synthesis of chiral compounds usually results in the synthesis of only one enantiomer of the two possible enantiomers. Synthetic, laboratory synthesis usually produces racemic mixtures of chiral compounds. |
|
|
Two Tetrahedral Stereocenters with Different Sets of Substituents
In 2,3-pentanediol, there are 22 = 4 stereoisomers differing from one another in the configurations at C2 and C3: The vertical bond between C2 and C3 in these four structures can be considered to be in the plane of the paper, with the bonds to C1 and C4 extending below the plane of the screen to the two methyl carbons. Show me? |
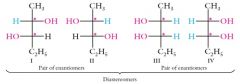
|
|
|
Explain the properties of compounds containing two or more tetrahedral stereocenters?
|
All diastereomers (except meso compounds, described next, and cis/trans compounds) are optically active.
Unlike enantiomers, diastereomers have specific rotations that usually differ in their numerical values and may have the same or opposite directions of rotation. Diastereomers differ in other physical properties such as MP, BP, and solubility. They also usually differ in their chemical properties. |
|
|
The artificial sweetener aspartame (NutraSweet), N-L-aspartyl-L-phenylalanine methyl ester, contains 2 stereocenters. Only the L,L stereoisomer is sweet.
Show me? |
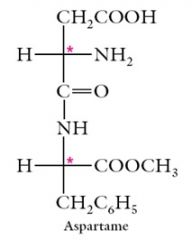
|
|
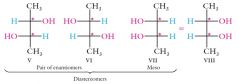
|
The third stereoisomer (VII = VIII) is called a meso compound. It is not an enantiomer of V or VI.
V and VI are diastereomers of the meso compound and vice versa. Meso compounds are achiral and optically inactive even though they contain two tetrahedral stereocenters. |
|
|
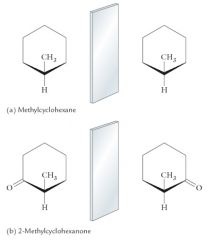
Cyclic Compounds Containing Tetrahedrals Steroecenters
The two nonring substituents are different. The two halves of the ring on each side of C1 are the same. There is no tetrahedral stereocenter. The two nonring substituents are different. The two halves of the ring on each side of C2 are different. C2 is a stereocenter. 2-Methylcyclohexanone exists as a pair of enantiomers. |
|
|
Isomers
• Isomers are different compounds that have what? |
Isomers
• Isomers are different compounds that have the same molecular formula. |
|
|
There are two types of isomers: What are they?
|
There are two types of isomers: constitutional isomers and stereoisomers.
|
|
|
Constitutional (structural) isomers differ in connectivity—explain?
|
Constitutional (structural) isomers differ in connectivity—that is, in the order of attachment of atoms to one another.
|
|
|
Stereoisomers have the same connectivity but differ in configuration—explain?
|
Stereoisomers have the same connectivity but differ in configuration—that is, the relative orientations in space of the atoms of a compound, independent of changes due to rotation about single bonds.
|
|
|
Stereoisomers that are mirror images of each other are called what?
|
Stereoisomers that are mirror images of each other are called enantiomers.
|
|
|
Diastereomers are stereoisomers (including geometrical isomers) that are not enantiomers—explain?
|
Diastereomers are stereoisomers (including geometrical isomers) that are not enantiomers—that is, they are not mirror
images of each other. |
|
|
Enantiomers
• A pair of enantiomers—nonsuperimposable mirror-image compounds—exists when ? |
Enantiomers
• A pair of enantiomers—nonsuperimposable mirror-image compounds—exists when there is a tetrahedral stereocenter, a carbon with four different substituents. |
|
|
Such compounds are also called ?
|
Such compounds are also called chiral compounds.
|
|
|
Enantiomers can be differentiated by a naming system that uses the prefixes what?
|
Enantiomers can be differentiated by a naming system that uses the prefixes D- and L-.
|
|
|
Chiral compounds are optically active:explain?
|
Chiral compounds are optically active: they rotate plane-polarized light, as measured by a polarimeter.
|
|
|
Each enantiomer in a pair of enantiomers rotates plane-polarized light the same number of degrees but in what directions?
|
Each enantiomer in a pair of enantiomers rotates plane-polarized light the same number of degrees but in opposite directions.
|
|
|
A racemic mixture—is a what?
|
A racemic mixture—an equimolar mixture of enantiomers—is optically
inactive. |
|
|
Enantiomers have the same chemical reactivity when
reacting with achiral reactants and what properties? |
Enantiomers have the same chemical reactivity when
reacting with achiral reactants but often show very large differences in reactivity when reacting with chiral reactants or in reactions catalyzed by chiral enzymes, a phenomenon called chiral recognition or chiral discrimination. |

