![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
48 Cards in this Set
- Front
- Back
|
Carboxylic acids contain a carbonyl group, C=O, with an attached –OH group. The entire functional group is called a carboxyl group and is often abbreviated –COOH or CO2H.
|
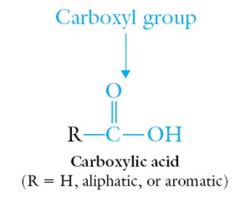
|
|
|
.
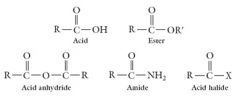
The families of carboxylic acid derivatives are the esters, acid anhydrides, amides, and acid halides: |

|
|
|
Explain some Carboxylic Acids and Derivates
|

|
|
|
All four types of carboxylic acid derivatives can be synthesized from carboxylic acids and hydrolyzed back to them.
The –OH, -OR’, -NH2, -O-CO-OR, or halogen group must be directly attached to the carbonyl carbon to be a carboxylic acid derivative: |
|
|
|
Constitutional isomers for carboxylic acids and their derivatives can be based on different carbon skeletons, or on different placements of the carboxyl group.
The orbital hybridization of the carbonyl carbon and oxygen atoms is sp2 as in the aldehydes and ketones. The bond angles about the carbonyl group are 120°. The atoms connected to the carbonyl group (C,N,O) are all sp3 hybridized with tetrahedral (109.5°) bond angles. The oxidation state of carbon in a carboxylic acid is the highest of the organic compounds of carbon: |
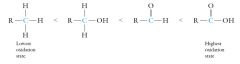
|
|
|
The synthesis of carboxylic acids
Benzoic acid is the simplest aromatic carboxylic acid. It can be prepared by selective oxidation of alkyl benzenes: |
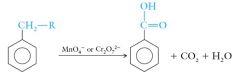
|
|
|
Cholinesterase inhibitor poisoning
symps |

you DUMBBEL ASS.
Diarrhea, Urination, Miosis, Bronchospasm, Bradycardia, Excitation of skeletal muscle and CNS, Lacrimation, Abdominal cramping, Sweating, and Salivation |
|
|
What are the rules of naming carboxylic acids.
|

|
|
|
Many carboxylic acids are referred to by their common names. The common name prefixes are the same as for the corresponding aldehydes.
|

|
|
|
What are some physical properties of Carboxylic acids.
Carboxylic acids have higher secondary forces than alcohols, aldehydes, or ketones, and consequently have higher boiling and melting points. |
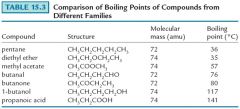
|
|
|
Both the carbonyl group and the hydroxyl group in a carboxylic acid are polar and capable of hydrogen bonding.
|
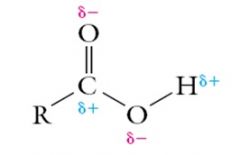
|
|
|
The presence of these two groups allows carboxylic acids to form hydrogen bonded dimers under most conditions:
|
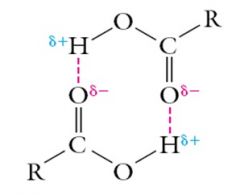
|
|
|
Carboxylic acids show slightly higher solubility in water than do alcohol because they can form three hydrogen bond interactions with water:
The solubility of carboxylic acids decreases more slowly than that of alcohols as the number of carbons increases. |
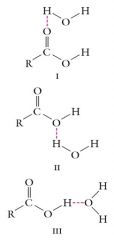
|
|
|
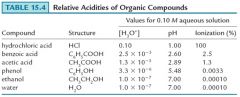
Acidity of Carboxylic Acids
Carboxylic acids are weaker acids than the strong acids (HCl, H2SO4, HNO3, and HClO4), but stronger acids than phenols, and much stronger than alcohols. |
|
|
|
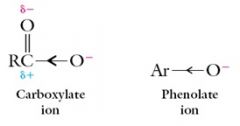
The larger acidity of carboxylic acids compared to phenol is due to the greater electron withdrawing power of the carbonyl group, compared to a benzene ring, which allows it to spread the negative charge out more.
|
|
|
|
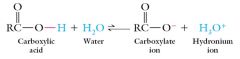
Carboxylic acids are weak acids that ionized to form carboxylate ion and hydronium ion in water.
The extent of ionization is less than 1-2%. The equilibrium lies to the left. |
|
|
|
Carboxylic acids are acidic enough to turn blue litmus paper red which distinguishes them from alcohols, but not phenols which also turn blue litmus paper red.
|

|
|
|
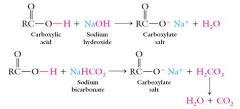
Sodium hydroxide and sodium bicarbonate both react with carboxylic acids to form a carboxylate salt:
|
|
|
|
A carboxylate salt is the product of a reaction between a carboxylic acid and a strong base.
The sodium salt of butanoic acid would be called sodium butanoate. |

|
|
|
Soaps and their Cleaning Properties
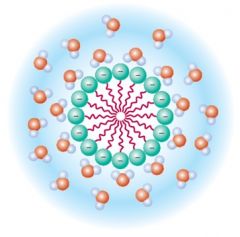
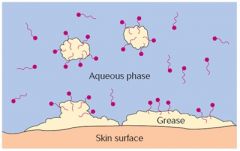
Dirt adheres to surfaces rather than dissolving in water because it is hydrophobic or physically combined with a hydrophobic substance such as body oils, cooking fats, or greases. Soaps solubilize greasy dirt because they are amphipathic—that is, they contain both water-loving (hydrophilic) and water-hating (hydrophobic) parts. |
|
|
|
The hydrophobic tail of a soap molecule has an affinity for the greasy dirt; the hydrophilic head, for water:
The soap molecules, helped by mechanical agitation, break up the greasy dirt into smaller fragments and surround them, presenting a hydrophilic surface to the surrounding water. |
|
|
|
The requirements for a good soap are what?
|
The requirements for a good soap are an ionic group as the hydrophilic head and a long hydrocarbon group as the hydrophobic tail.
Stearic acid (C18) does not function as a soap, because it does not possess an ionic head group. Its carboxylate salt is a good soap, however. Sodium butyrate, CH3(CH2)2COO-Na+, does not function as a soap because its hydrocarbon tail is not long enough (not sufficiently hydrophobic). |
|
|
Esters from Carboxylic Acids and Alcohols
When an acid is heated with an alcohol (R’ = aliphatic) or a phenol (R’ = aromatic) in the presence of a strong acid catalyst, an ester is formed. This process is called esterification. |
|
|
|
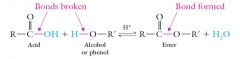
Esters from Carboxylic Acids and Alcohols
When an acid is heated with an alcohol (R’ = aliphatic) or a phenol (R’ = aromatic) in the presence of a strong acid catalyst, an ester is formed. This process is called esterification. |
|
|
|
What are some properties of esters and estirification?
|
Esterification is a reversible reaction. Removal of water shifts the equilibrium to the right.
Esters in living organisms are synthesized by reactions catalyzed by enzymes. Esters can also be formed from the reaction of an alcohol (or phenol) with a carboxylic acid anhydride or halide, rather than a carboxylic acid. Esters of carboxylic acids have pleasant odors and flavors: Pineapple ethyl butanoate Raspberry isobutyl methanoate Banana 3-methyl-butyl ethanoate Various esters are used to create the fragrances of perfumes. |
|
|
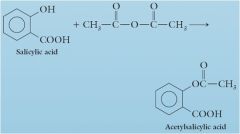
Aspirin (acetylsalicylic acid) is an ester derived from p-hydroxybenzoic acid (salicylic acid) and acetic acid.
|
|
|
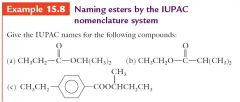
|
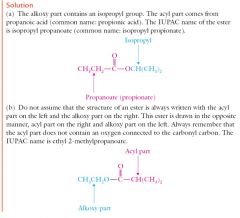
|
|
|
What are some of physical properties of esters.
|
Esters have much lower boiling and melting points than those of carboxylic acids. Ester molecules cannot hydrogen bond to each other.
Propanoic acid 141°C Methyl acetate 57°C Esters are less soluble in water than are carboxylic acids because esters cannot form as many hydrogen bonds to water molecules as can carboxylic acids. The secondary forces in esters are weaker than those in aldehydes and ketones, and thus esters have lower melting and boiling points. Methyl acetate 57°C Butanal 76°C Esters have about the same water solubilities as aldehydes and ketones because all three hydrogen bond to water equally well. |
|
|
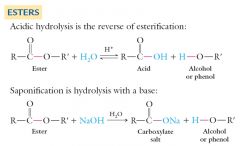
The hydrolysis of an ester to the corresponding carboxylic acid and alcohol (or phenol) occurs under either acidic or basic conditions.
Acidic hydrolysis requires the presence of a strong acid: |
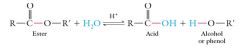
|
|
|
Acidic hydrolysis of an ester is the reverse of the acid catalyzed formation of an ester. Both are equilibrium reactions.
The equilibrium can be shifted to favor hydrolysis by carrying out the reaction with a large excess of water. Physiological synthesis and hydrolysis of esters are carried out by special proteins called enzymes, rather than by strong acid catalysis. Basic hydrolysis requires a strong base such as NaOH or KOH: This reaction is also called saponification, and is used to manufacture soaps from certain animal fats and vegetable oils. |
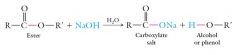
|
|
|
Acidic and basic hydrolysis differ in two important ways
|

|
|

|

|
|
|
What are some properties of Carboxylic Acid Anhydrides and Halides?
|
Carboxylic acid anhydrides are named by changing the word acid to anhydride in the name of the carboxylic acid.
Acid halides (acyl halides) are named by changing the name of the carboxylic acid from –ic acid to –yl halide. |
|
|
Acid anhydrides react with alcohols (or phenols) to form esters
Ester synthesis from anhydrides or acid halides is more efficient than from the carboxylic acid itself. |
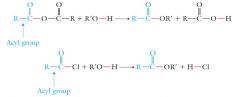
|
|
|
What are acyl transfer agents and its properties?
|
Acid anhydrides and halides, as well as carboxylic acids, are often called acyl transfer agents because they transfer the acyl group, RCO, to the oxygen of an alcohol (or phenol).
Acyl transfer reactions are biologically important: Protein synthesis occurs through an acyl transfer reaction. Coenzyme A thioesters participate in metabolic reactions through acyl transfer reactions. |
|
|
Acids other than carboxylic acids can form anhydrides. Phosphoric acid can form both diphosphoric acid and triphosphoric acid:
|
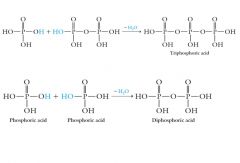
|
|
|
Phosphoric, diphosphoric, and triphosphoric acids have what properties and why?
|
Phosphoric, diphosphoric, and triphosphoric acids have acidic properties somewhat stronger than those of carboxylic acids, but weaker than those of strong acids, such as HCl, H2SO4, HNO3, and HClO4.
Each O-H group in phosphoric, diphosphoric, and triphosphoric acid is acidic. All three acids are polyprotic acids. |
|
|
Diphosphoric and triphosphoric acids are anhydrides as well as acids
|
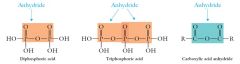
|
|
|
Phosphoric, diphosphoric, and triphosphoric acids can all undergo esterification reactions to form phosphoric acid esters or phosphate esters.
|
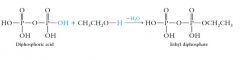
|
|
|
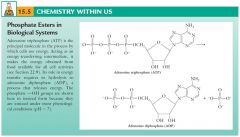
Many phosphate esters are biologically important:
ATP, NADH and NAD+, phospholipids, DNA and RNA (nucleic acids), and phosphate esters of carbohydrates. |
|
|
|
Carboxylic Acids and Their Derivatives Compared
• Carboxylic acids contain the carboxyl group, -COOH, which is a carbonyl group with an -OH group attached to it. • Carboxylic acids and their derivatives differ in the group attached to the carbonyl carbon. |
|
|
|
Carboxylic Acids and Their Derivatives Compared – cont.
|
Carboxylic Acids and Their Derivatives Compared – cont.
• Amides have nitrogen (in an –NH2, -NHR, or –NR2 group), and acid halides have a halogen connected to the carbonyl carbon. |
|
|
Explain the properties of Carboxylic acids.
|
• Carboxylic acids are synthesized by the selective oxidation of alkylbenzenes, primary alcohols, and aldehydes.
• Carboxylic acids are named in the IUPAC system by naming the longest continuous carbon chain containing the carboxyl group. • The ending of the corresponding alkane is changed from -e to -oic acid. • The carboxyl carbon is C1. • Prefixes preceded by numbers indicate substituents attached to the longest continuous chain. • C6H5COOH is called benzoic acid. • Carboxylic acids have considerably higher boiling and melting points than those of alcohols because of dimer formation by hydrogen bonding between pairs of acid molecules. |
|
|
Continue to explain the properties of carboxylic acids.
|
Carboxylic acids have good water solubility, slightly higher than that of alcohols, owing to strong hydrogen-bond attractions with water.
• Carboxylic acids are weak acids but are stronger acids than phenols and much stronger acids than alcohols. • Carboxylic acids and phenols can be distinguished from alcohols by litmus paper. |
|
|
Explain the properties of Carboxylate Salts.
|
• Carboxylate salts, formed by the reaction of carboxylic acids with strong base, are named by the name of the positive ion followed by the name of the carboxylic acid, with the ending changed from -ic acid to -te.
• Carboxylate salts are ionic compounds and, therefore, have much higher melting and boiling points and water solubility than those of carboxylic acids. • Sodium and potassium carboxylate salts derived from unbranched long-chain acids with 12 to 20 carbons are used as soaps. |
|
|
Explain the properties of Carboxylic Acid Anhydrides and Halides.
|
• Carboxylic acid anhydrides and halides undergo hydrolysis to form esters.
• They react with alcohols and phenols to form esters. |
|
|
Explain the properties of Phosphoric acids and their derivatives.
|
Phosphoric, diphosphoric, and triphosphoric acids are stronger acids than carboxylic acids but are weaker than the strong acids, such as HCl, H2SO4, HClO4, and HNO3.
• Like carboxylic acids, phosphoric acids undergo esterification with alcohols or phenols. |
|

|
|

