![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
alkenes are sometimes called
|
olefin
|
|
|
alkene
|
a hydrocarbon that contains a carbon-carbon double bond
|
|
|
degree of unsaturation
|
the number of rings and/or multiple bonds present in the molecule
|
|
|
organohalogen compounds
|
C,H, X, where X = F, Cl, Br, or I
|
|
|
organooxygen compounds
|
C, H, O
|
|
|
organonitrogen compounds
|
C, H, N
|
|
|
Valium is made from:
|
diazepam
|
|
|
Valium
|
antianxiety medication
|
|
|
If more than one double bond is present, what suffix should be added?
|
-diene, triene, et cetera
E.g., 1,3-butadiene |
|
|
ethylene
|
the common name for ethene
|
|
|
propylene
|
common name for propene
|
|
|
isobutylene
|
common name for 2-methylpropene
|
|
|
isoprene
|
common name for 2-methyl-1,3-butadiene
|
|

Name these:
|

Hint: Remember "zeh zame zide"
|
|
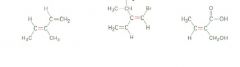
Name 'em
|
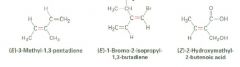
|
|
|
Which is more stable, a tridistributed or didistributed double bond?
|
tri-distributed
|
|
|
Markovnikov's rule
|
In the addition of HX to an alkene, the H attaches to he carbon with fewer alkyl substituents and the X attached to the carbon with more alkyl substituents
|
|
|
Hammond postulate
|
transition states represent energy maxima; they are high-energy activated complexes that occur transiently during the course of a reaction and immediately go on to a more stable species
|
|
|
hydride shift
|
the shift of a hydrogen atom and its electron pair between neighboring carbons
|
|
|
What plant hormone induces ripening in fruit?
|
ethylene
|
|
|
What pigment is responsible for the color of carrots?
|
β-carotene
|
|
|
What are the two most important organic chemical compounds produced industrially?
|
ethylene and propylene
|
|
|
Draw acetaldehyde
|
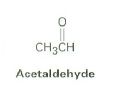
|
|
|
What is the common name for ethene?
|
ethylene
|
|
|
What is the common name for propene?
|
propylene
|
|
|
Does inserting an oxygen to form an organoxygen compound affect the formula of an equivalent hydrocarbon?
|
No, it can be ignored because oxygen forms two bonds.
|
|
|
Does inserting a nitrogen to form an organonitrogen compound affect the formula of an equivalent hydrocarbon?
|
Yes, because forms three bonds it adds one more hydrogen than a related hydrocarbon.
|
|
|
Draw isopropyl
|
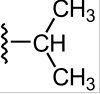
|
|
|
disubstituted
|
two substituents other than hydrogen are bonded to the double-bond carbons
|
|
|
heavy hydrogen is called:
|
deuterium
(hydrogen-2) |
|
|
protium
|
hydrogen-1, the most common isotope of the element hydrogen, with one proton and no neutrons
|

