![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
- 3rd side (hint)
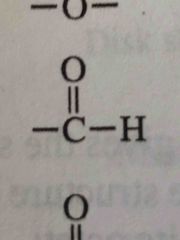
What functional group is this? |
Ketone |
|
|
|
Two types or classes of hydrocarbons |
Aliphatic Aromatic |
|
|
|
A hydrocarbon having only C-C single bonds |
Alkane |
|
|
|
A hydrocarbon having a carbon to carbon double bond |
Alkene |
|
|
|
Ethene or ethylene C2H4 is an |
Alkene |
|
|
|
A hydrocarbon having a C-C triple bond |
Alkyne |
|
|
|
ANE suffix ENE suffix YNE suffix |
Single C-C Bonds Double C-C bonds Triple C-C bonds |
|
|
|
Ethyne aka acetylene is an |
Alkyne (has triple carbon bonds) |
|
|
|
A part of a hydrocarbon molecule that has a group of atoms attached as a group |
Functional group |
|
|
|
-OH functional group |
Alcohol |
|
|
|
-O- functional group |
Ether |
|
|
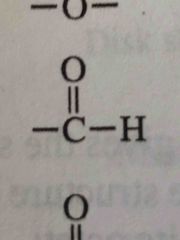
What functional group is this |
Aldehyde group |
|
|
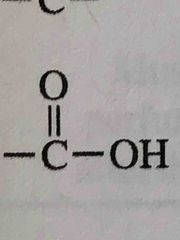
What functional group is this? |
Carboxylic acid |
|
|
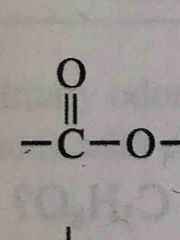
What functional group is this? |
Ester |
|
|
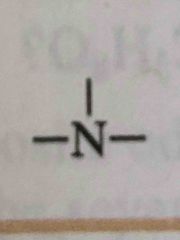
What functional group is this? |
Amine |
|
|
|
Example of a carboxylic acid |
Acetic acid |
Vinegar |
|
|
Example of a ketone |
Acetone |
Nail polish remover |
|
|
Example of an amine |
Methyl amine CH3NH2 used in dyes (ammonia smell) |
|
|
|
Example of an alcohol |
Methyl alcohol |
|
|
|
Example of an aldehyde |
Acetaldehyde |
|
|
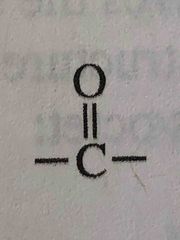
What functional group is this? |
Ketone |
|
|
|
1 carbon prefix |
Mono |
Monoxide pollutant |
|
|
2 carbon prefix |
Eth |
Ethane ripens your fruit |
|
|
3 carbon prefix |
Prop |
Propane gas grills |
|
|
4 carbon prefix |
But |
Butane cigarette lighter |
|
|
5 carbon prefix |
Hept |
Heptane mostly used as a solvent |
|
|
A covalent bond that involves the sharing of one pair of electrons |
Single bond |
|
|
|
Representation of a compound consisting of the symbols of the component elements , each surrounded by dots and showing shared and unshared electrins |
Lewis structure |
|
|
|
Bonding between two atoms resulting from the sharing of electrons |
Covalent bond |
|
|
|
A hydrocarbon containing only C-C single bonds |
Alkane |
|
|
|
Bonding between two atoms resulting from the sharing of electrins |
Covalent bond |
|
|
|
A solid structure in which the ions are arranged in a regular repeating pattern |
Ionic crystal |
|
|
|
A rule stating that atoms tend to gain lose or share electrons to achieve an electron configuration with eight electrons in the valence shell |
Octet rule |
|
|
|
A measure of the ability of an atom to attract electrons within a bond to itself |
Electronegativity |
|
|
|
A covalent bond that involves the sharing of three pairs of electrons |
Triple bond |
|
|
|
A measure of the ability of an atom to attract electrons within a bond to itself |
Polarity |
|
|
|
A covalent bond that involves the sharing of three pairs of electrins |
Triple bond |
|
|
|
The repeating pattern of atoms or ions in a crystal |
Crystal lattice |
|

