![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
41 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Activation energy
|
The amount of energy that reactants must absorb before a chemical reaction will start
|
|
|
|
Active site
|
The specific portion of an enzyme that binds the substrate by means of multiple weak interactions and that forms the pocket in which catalysis occurs.
|
|
|
|
Adenosine triphosphate (ATP)
|
An adenine-containing nucleoside triphosphate that releases free energy when its phosphate bonds are hydrolyzed. This energy is used to drive endergonic reactions in cells.
|
|
|
|
Allosteric regulation
|
The binding of a regulatory molecule to a protein at one site that affects the function of the protein at a different site.
|
|
|
|
Anabolic pathway
|
A metabolic pathway that consumes energy to synthesize a complex molecule from simpler compounds.
|
|
|
|
Bioenergetics
|
(1) The overall flow and transformation of energy in an organism. (2) The study of how energy flows through organisms.
|
|
|
|
Catabolic pathway
|
A metabolic pathway that releases energy by breaking down complex molecules to simpler compounds.
|
|
|
|
Catalyst
|
A chemical agent that increases the rate of a reaction without being consumed by the reaction.
|
|
|
|
Chemical energy
|
Energy available in molecules for release in a chemical reaction
|
|
|
|
Coenzyme
|
An organic molecule serving as a cofactor. Most vitamins function as coenzymes in metabolic reactions.
|
|
|
|
Cofactor
|
Any nonprotein molecule or ion that is required for the proper functioning of an enzyme. Cofactors can be permanently bound to the active site or may bind loosely with the substrate during catalysis.
|
|
|
|
Competitive inhibitor
|
|
|
|
|
Cooperativity
|
A substance that reduces the activity of an enzyme by entering the active site in place of the substrate whose structure it mimics.
|
|
|
|
Endergonic reaction
|
A nonspontaneous chemical reaction, in which free energy is absorbed from the surroundings.
|
|
|
|
Energy
|
The capacity to cause change, especially to do work (to move matter against an opposing force).
|
|
|
|
Energy coupling
|
In cellular metabolism, the use of energy released from an exergonic reaction to drive an endergonic reaction.
|
|
|
|
Entropy
|
A measure of disorder, or randomness.
|
|
|
|
Enzyme
|
A macromolecule serving as a catalyst, a chemical agent that changes the rate of a reaction without being consumed by the reaction.
|
|
|
|
Enzyme-substrate complex
|
A temporary complex formed when an enzyme binds to its substrate molecule(s).
|
|
|
|
Exergonic reaction
|
A spontaneous chemical reaction, in which there is a net release of free energy.
|
|
|
|
Feedback inhibition
|
A method of metabolic control in which the end product of a metabolic pathway acts as an inhibitor of an enzyme within that pathway.
|
|
|
|
First law of thermodynamics
|
The principle of conservation of energy
|
|
|
|
Free energy
|
The portion of a biological system’s energy that can perform work when temperature and pressure are uniform throughout the system. (The change in free energy of a system is calculated by the equation ΔG = ΔH – TΔS, where H is enthalpy [in biological systems, equivalent to total energy], T is absolute temperature, and S is entropy.)
|
|
|
|
Heat
|
The total amount of kinetic energy due to the random motion of atoms or molecules in a body of matter
|
|
|
|
Hemoglobin
|
An iron-containing protein in red blood cells that reversibly binds oxygen.
|
|
|
|
Induced fit
|
Induced by entry of the substrate, the change in shape of the active site of an enzyme so that it binds more snugly to the substrate.
|
|
|
|
Kinetic energy
|
The energy associated with the relative motion of objects. Moving matter can perform work by imparting motion to other matter.
|
|
|
|
Metabolic pathway
|
A series of chemical reactions that either builds a complex molecule (anabolic pathway) or breaks down a complex molecule into simpler compounds (catabolic pathway).
|
|
|
|
Metabolism
|
The totality of an organism’s chemical reactions, consisting of catabolic and anabolic pathways, which manage the material and energy resources of the organism.
|
|
|
|
noncompetitive inhibitor
|
A substance that reduces the activity of an enzyme by binding to a location remote from the active site, changing the enzyme’s shape so that the active site no longer functions effectively.
|
|
|
|
Order
|
In classification, the taxonomic category above the level of family.
|
|
|
|
Phosphorylated
|
Referring to a molecule that is covalently bonded to a phosphate group.
|
|
|
|
Potential energy
|
The energy that matter possesses as a result of its location or spatial arrangement (structure).
|
|
|
|
Ribose
|
The sugar component of RNA nucleotides.
|
|
|
|
Second law of thermodynamics
|
The principle stating that every energy transfer or transformation increases the entropy of the universe. Ordered forms of energy are at least partly converted to heat.
|
|
|
|
Substrate
|
The reactant on which an enzyme works.
|
|
|
|
Thermal energy
|
See heat.
|
|
|
|
Thermodynamics
|
The study of energy transformations that occur in a collection of matter. See first law of thermodynamics and second law of thermodynamics.
|
|
|
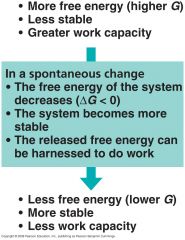
The relationship of free energy to stability, work capacity, and spontaneous change.
|
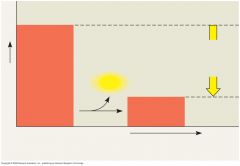
Usable systems (top diagrams) are rich in free energy, G. They have a tendency to change spontaneously to a more stable state (bottom), and it is possible to harness this "downhill" change to perform work.
|
Gravitational motion: Objects move spontaneously from a higher altitude to a lower one.
Diffusion: Molecules in a drop of dye diffuse until they are randomly dispersed. Chemical reaction: In a cell, a sugar molecule is broken down into simpler molecules. |
|
Exergonic reaction
|
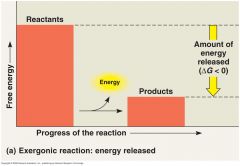
Proceeds with a net release of free energy.
o Because chemical mixture loses free energy (G decreases). o ∆G is negative for an exergonic reaction, using ∆G as a standard for spontaneity; exergonic reactions are those that occur spontaneously. (Remember that the word spontaneous does not imply that a reaction will occur instantaneously or even rapidly.) • The magnitude of ∆G for an exergonic reaction represents the maximum amount of work the reaction can perform. o The greater the decrease in free energy, the greater the amount of work that can be done. |
Exergonic and Endergonic Reactions in Metabolism
Based on free energy changes, chemical reactions can be classified as either exergonic (“energy outward”) or endergonic (“energy inward”). |
|
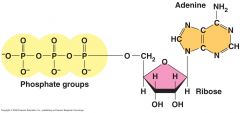
The structure of adenosine triphosphate (ATP).
In the cell, most hydroxyl groups of phosphates are ionized (-O). |
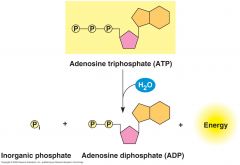
The hydrolysis of ATP.
The reaction of ATP and water yields inorganic phosphate and ADP and releases energy. |
|

