![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
46 Cards in this Set
- Front
- Back
|
Chirality |

- property of having handedness - chiral objects cannot be superposed on its mirror image |
|
|
Stereoisomers |
- atoms connected in same sequence, but differ in arrangement of atoms in space - Ex: cis and trans forms of alkenes - Stereoisomers are not constitutional isomers - divided into 2 categories: enantiomers and diastereomers |
|
|
Enantiomers |
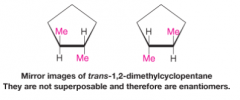
- stereoisomers whose molecules are nonsuperposable mirror images of each other - occur only with compounds whose molecules are chiral |
|
|
Diastereomers |
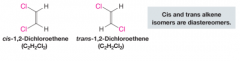
- stereoisomers whose molecules are not mirror images of each other - have different physical properties (melting and boiling points, soluability) |
|
|
Subdivision of Isomers |
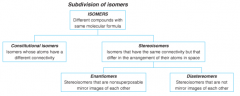
|
|
|
Chiral Molecule |
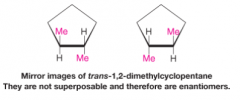
- molecule that is not superposable on its mirror image - have handedness - capable of existing as a pair of enantiomers |
|
|
Achiaral Molecule |

- superposable on its mirror image |
|
|
Chirality of 2-Butanol |
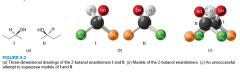
- not superposable - mirror images of each other |
|
|
Chiral Specificity of Enantiomers |
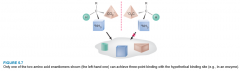
- the binding specificity for a chiral molecule at a chiral receptor site is only favorable in one way. |
|
|
Determining Chirality of a Molecule |
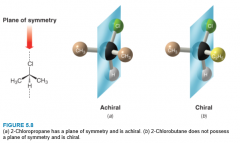
- A molecule will not be chiral if it possesses a plane of symmetry - All molecules with a plane of symmetry in their most symmetric conformation are achiral. |
|
|
R,S-System |
- A method for designating the configuration of tetrahedral chirality centers. |
|
|
Configurations |
- The particular arrangement of atoms (or groups) in space that is characteristic of a given stereoisomer |
|
|
Assigning (R) and (S) Configurations - Priority |
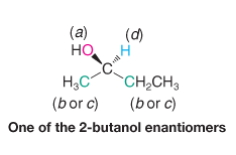
- assign priority as a,b,c, or d - higher atomic number of atom directly attached to chiral carbon, the higher priority |
|
|
Assigning (R) and (S) Configurations - Priority at First Point of Difference |
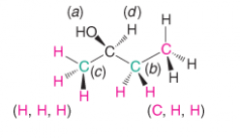
- when priority cannot be assigned on the basis of atomic number directly attached to chiral C - next set of atoms in unassigned group is examined for atomic mass - continue until point of first difference |
|
|
Assigning (R) and (S) Configurations |
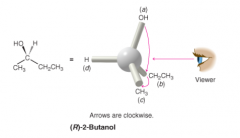
- rotate the molecule so that lowest priority is away from you - trace path from a to b to b - (R) if path is clockwise - (L) if path is counterclockwise |
|
|
Assigning (R) and (S) Configurations - Compounds Containing Multiple Bonds |
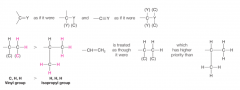
- Groups containing double or triple bonds are assigned priorities as if both atoms were duplicated or triplicated |
|
|
Pure Enantiomers |
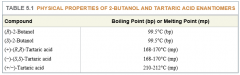
- enantiomers with identical: - melting and boiling points - idex of refraction - soluability - IR spectra - rates of reaction with achiral reagents - only show different behavior when they interact with other chiral substances, including their own enantiomers |
|
|
Optically Active Compounds |
- compound that rotates the plane of polarized light - separate enantiomers rotate the plane of polarized light in equal amounts but in opposite directions - Achiral molecules are not (in solution cause no rotation of plane of polarized light) but oriented achiral molecules and crystals with specific symmetric characteristics can - Chiral molecules are |
|
|
Polarimeter |
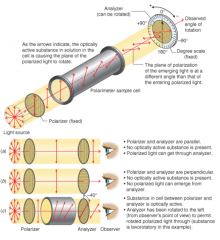
- Achiral molecules in solution cause no rotation of plane of polarized light |
|
|
Specific Rotation |

-constant calculated from observed rotation of compound from polarimeter - depends on Temp and wavelength, which is added outside of brackets |
|
|
Nomenclature with Specific Rotaion |
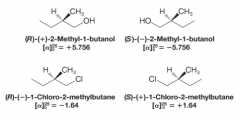
- no correlation exists between (R) and (S) configurations and the direction (+) or (-) in which they rotate light |
|
|
Racemic Mixture |
- equimolar mixture of two enantiomers - causes no rotation of plane polarized light - designated with (±) |
|
|
Enantiomerically Pure |
- enantiomeric excess of 100% |
|
|
Optical Purity |

- entiomeric excess (ee) - calculated from optical rotation or moles of enantiomers - means x percent of the mixture consists of the excess enantiomer and x percent of mixture consists of the racemic form |
|
|
Synthesis of 2-butanol by Hydrogenation of butanone |
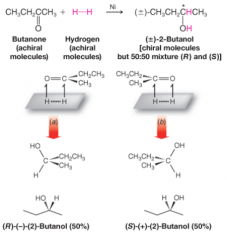
- nickel catalyzed - achiral reactants lead to chiral products - since butanol is achiral, two faces of trigonal planer carbonyl group interact with metal surface with equal probability - reaction produces a racemic mixture in the absence of any chiral influence from catalyst, reagent, or solvent |
|
|
Stereocentric Reactions |
- reactions that lead to a preferential formation of one stereoisomer over other stereoisomers that could be formed - requires chiral agent, catalyst, or solvent to assert influence on the course of reaction |
|
|
Enantioselective Reaction |
- If a reaction produces preferentially one enantiomer over its mirror image
|
|
|
Diastereoselective Reaction |
- If a reaction leads preferentially to one diastereomer over others that are possible |
|
|
Hydrolysis |
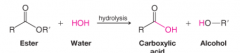
- ester reacts with a molecule of water to produce a carboxylic acid and an alcohol - lipase catalyzes this reaction |
|
|
Kinetic Resolution |
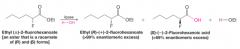
- rate of reaction with one enantiomer is different than with the other, leading to a product with a majority of one stereoisomer - EX. Lipase catalyzed hydrolysis reaction - lipase reacts selectively with one ester enantiomer, while other enantiomer is unchanged and reacts slower
|
|
|
Rule for MAx # of Stereoisomers |
- in compounds where stereoisomerism is due to chirality centers - total number of stereoisomers will not exceed 2^n, where n= # of chirality centers - total number can be less than this |
|
|
Drawing Stereoisomers for Molecules with More than One Chirality Center |
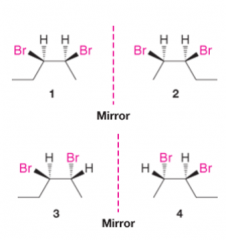
- draw portion of carbon skeleton that contains chirality centers so that as many of the chirality centers in the plane of the paper as possible, and as symmetrically as possible - add the remaining groups that are bonded at the chirality centers in such a way as to maximize the symmetry between the chirality centers (1) - draw the enantiomer of the first stereoisomer, draw its mirror image (2) - draw another stereoisomer, we interchange two groups at any one of the chirality centers (3 and 4) |
|
|
Analysis of Stereoisomers of 2,3-Dibromopentane |
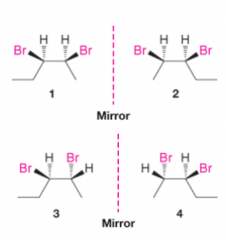
- 1-4 are all optically active - 1 and 2 are enantiomers - 3 and 4 are also enantiomers - 1 and 3 are diastereomers |
|
|
Meso Compounds |
- achiral molecule that contains chirality centers and has an internal plane of symmetry - not optically active |
|
|
Naming Compounds with More than One Chirality Center |
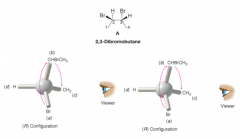
- analyze each chiral center separately - tell which designation refers to which C atom - compound is (2R,3R)-2,3-dibromobutane |
|
|
Fischer Projections |
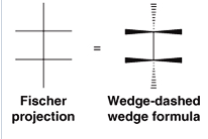
- show 3 dimensions in chiral molecules like acyclic carbohydrates - useful in cases where there are chirality centers at several adjacent Carbon atoms - flipping projection produces enantiomer |
|
|
Drawing Fischer Projections |
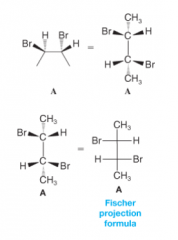
- carbon chain in a Fischer projection is always drawn from top to bottom - consider the molecule has eclipsing interactions between the groups at each carbon - “project” all of the bonds onto the paper, replacing all solid and dashed wedges with ordinary lines. - projections can be rotated in the plane of the paper by 180 degrees but by no other angle |
|
|
1,4-Dimethylcyclohexanes |
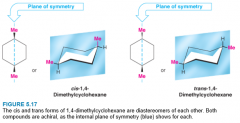
- no chirality centers, but has 2 stereogenic center - cis-trans isomers are diastereomers - not optically active |
|
|
1,3-Dimethylcyclohexanes |
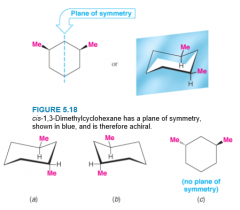
- 2 chirality centers - only 3 stereoisomers - cis-1,3-Dimethylcyclohexane is achiral - trans-1,3-Dimethylcyclohexane is chiral and exists as a pair of enantiomers |
|
|
1,2-Dimethylcyclohexanes |
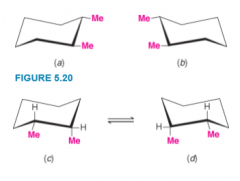
- 2 chirality centers, however can only isolate 3 stereoisomers - cis-1,2-Dimethylcyclohexane is chiral, however a ring flip interconverts one enantiomer to the other at normal temperatures. Cannot isolate (conformational stereoisomers) |
|
|
Retention of Configuration |
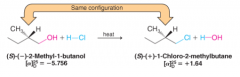
- if no bonds in stereogenic center are broken, configuration is retained through reaction - R,S designation can change - optical rotation can change |
|
|
Relative Configuration |
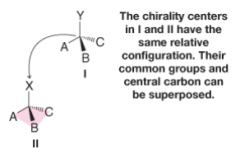
- stereogenic centers in different molecules share 3 groups in common - central carbon can be superimposed in a pyramidal arrangement |
|
|
Absolute Configuration |
- The actual arrangement of groups in a molecule. |
|
|
Chirality Center (Stereogenic Center) |
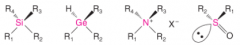
- An atom bearing groups of such nature that an interchange of any two groups will produce a stereoisomer. - any tetrahedral atom with four different groups attached to it has one |
|
|
Atropisomers |
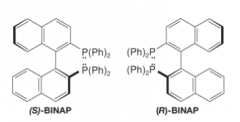
- Conformational isomers that are stable, isolable compounds. - In BINAP restricted rotation about the bond between naphthalene rings causes chirality |
|
|
Allenes |
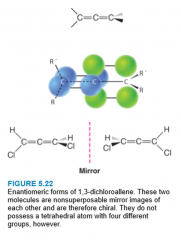
- compounds that exhibit stereoisomerism - planes of pi bonds are perpendicular to each other - allenes with different substituents on the end carbon atoms are chiral - do not show cis-trans isomerism |

