![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
51 Cards in this Set
- Front
- Back
|
Chemical Evolution |
The theory that states that simple chemicals came together to form larger, more complex ones. The complexity continued until it formed life. |
|
|
Oparin-Haldane Proposal |
1. The reactions of H2, N2, NH3, and CO2 led to more complex componds 2. Simple organic compounds came together to form larger molecules (i.e. amino acids, nucleotides, and sugars). 3. Further complexity adds to create proteins, nucleic acids, and complex carbohydrates. 4. Life became possible when one of these complex molecules could self-replicate. |
|
|
Scientific Theory |
A well-substantiated explanation of some aspect of the natural world that is acquired through the scientific method and repeatedly tested and confirmed through observation and experimentation. |
|
|
Prebiotic (Primordial) Soup Model |
Typically in reference to the early oceans, ponds, lakes, or any body of water where small molecules reacted with others in close contact with organic compounds. |
|
|
Proteins |
A macromolecule consisting of one or more polypeptide chains composed of 50 or more amino acids linked together. Each protein has a unique sequence of amino acids. Usually refers to the complete, often functional form. |
|
|
Amino Acids |
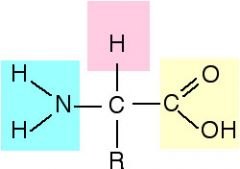
The simple sub-unit of a protein. |
|
|
Side Chain |
Another name for the R-group. |
|
|
R-group |
Unique structure of an amino acid (20 different kinds). Properties of amino acids vary because of the R-group. Attached to alpha carbon. |
|
|
Functional group |
A group of atoms responsible for the characteristic reactions of a particular compound. In amino acids this is in reference to either the amino, carboxyl, or special R- group. |
|
|
Amino group |
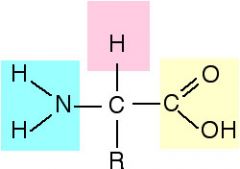
NH2 |
|
|
Carboxyl group |
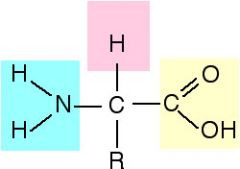
-COOH |
|
|
Sulfhydryl group |
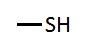
Located on R-group. Can form disulfide (S--S) bonds between other sulfhydrl groups. |
|
|
Hydroxyl group |
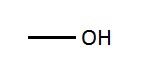
Usually relates to polarity of the amino acid. |
|
|
Hydrophobic |
Do not interact with water. Amino acids that are nonpolar usually have a lot of carbon boded to hydrogen, and few have sulfur. Tendency to clump together in water. |
|
|
Hydrophilic |
Polar or charged side chains interact readily with water. Contains a oxygen on the R-group or some charged atom. |
|
|
Polar |
A molecule containing a partial positive and partial negative charge on either side. O--H N--H |
|
|
Nonpolar |
A bond where electrons are equally shared.
|
|
|
Monomer |
A single sub unit. Examples include amino acids, nucleotides, or a sugar. For proteins this is just a single amino acid. |
|
|
Polymer |
More than one monomer bound together. For proteins this involves polymerization of amino acids. |
|
|
Polymerization |
The process by which small monomers are covalently bonded together to form polymers. |
|
|
Macromolecules |
Very large molecules that are made of smaller ones that are joined together in a chain. |
|
|
Oligopeptide (Peptide) |
Fewer than 50 amino acids linked together. |
|
|
Polypeptide |
50 or more amino acids linked together. |
|
|
Endergonic |
Referring to a chemical reaction that requires the input of energy to occur, where the products are greater free energy than the reactants. |
|
|
Exergonic |
Referring to the release of energy or increasing entropy. |
|
|
Condensation reaction (dehydration reaction) |
A chemical reaction in which two molecules are joined covalently with the removal or water as a byproduct. |
|
|
Hydrolysis |
The reverse of condensation reactions, here polymers are broken by adding water. The resulting monomer is separated from a polymer. |
|
|
Peptide bond |
In a protein polymer it is the amino group bonded to the carboxl group through a condensation reaction. |
|
|
Residue |
Refers to a specific monomer in the polypeptide chain. |
|
|
N-terminus |
The starting of a polypeptide chain where there is a "free" amino group. |
|
|
C-terminus |
The tail of a polypeptide chain where there is a "dangling" carboxyl group. |
|
|
catalysis |
Something that speeds up a reaction, but is not used up in the process. |
|
|
enzyme |
A protein catalyst used by living organisms to speed up and control biological reactions. |
|
|
Primary structure |
A complete polypeptide chain. |
|
|
Secondary structure |
Folding of primary structure held together by H-bonds. |
|
|
Alpha helix |
A type of secondary structure of proteins that is coiled. Held by H-bonds. |
|
|
Beta pleated sheets |
A type of secondary structure of proteins where segments fold in 180 degree bends that also fold in planes. |
|
|
Tertiary structure |
Further complexity of secondary structures of proteins where the R-groups interact with other R-groups or the back-bone of the peptide itself. |
|
|
Van der Waals interactions |
A temporary partial charge interactions that involve the hydrophobic R-groups of a peptide. Associated with tertiary structures. |
|
|
Disulfide bonds |
Form between two cysteines through a reaction between the sulfhydryl groups. Associated with tertiary structures. |
|
|
Quaternary structures |
multiple polypeptides that come together in a particular arrangement. Involves multiple tertiary structures acting as one. |
|
|
Dimer |
A chemical compound composed of two identical or similar monomers. |
|
|
Tetramer |
A polymer containing four identical monomers. |
|
|
Multienzyme complex |
A structurally distinct and ordered collection of enzymes, often catalyzing successive steps in a metabolic pathway. |
|
|
Denaturation |
Unraveling of protein structure by some external stress (strong acid/base, organic solvent) |
|
|
Renaturation |
The refolding of a protein to its native form. Only few proteins can do this. |
|
|
Molecular Chaperone |
A protein that facilitates the folding of newly synthesized proteins into their correct shape. Uses ATP in the process. |
|
|
Prion |
Proteins that are incorrectly folded and result in an infectious, disease causing agent. Can lack nucleic acids. |
|
|
Substrates |
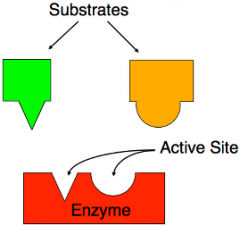
A reactant that interacts with a catalyst, such as an enzyme or ribozyme, in a chemical reaction. |
|
|
Lock-and-Key model. |
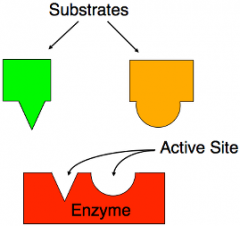
The interaction between a substrate and enzymes where the fit determines if the reaction takes place. |
|
|
Active Site |
The place where substrates bind and interact. This is where catalysis actually take place. |

