![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
_____ is a measure of an atom’s electron “hogginess” when sharing electrons in a covalent bond.
|
Electronegativity
|
|

What is represented by the numbers in blue?
Do we need to memorize these values? |
The numbers represent the electronegativity values for each element.
We do NOT need to memorize these, as they will be provided for us on the test. |
|
|
_____ _____ _____ occur when a bond is covalent and the electronegativity of the bonded atoms does not match. In turn, the electrons in that bond will be shared unequally.
|
Polar covalent bonds
|
|
|
What must be assigned to each atom in a polar covalent bond?
|
Partial positive/negative charges.
|
|
|
What symbol is used to indicate a partial positive or negative charge in a covalent bond?
|

The small Greek symbol for Delta (see the picture).
|
|
|
When a bond is covalent, and the electrons are shared equally, this is called a _____ _____ _____.
|
Nonpolar covalent bond
|
|
|
Covalent bonds are polar unless one of two things is true. Name them.
|
1) The atoms being bonded are the same.
2) C and H are bonding. |
|
|
What determines whether a molecule is polar or nonpolar?
|
The polarity of bonds will partially determine whether a structure is polar or nonpolar.
|
|
|
The polarity of molecules will play a major role in determining what four physical properties of a compound?
|
1) Melting Point
2) Boiling Point 3) Density 4) Water Solubility |
|
|
The shape of a molecule is determined by the bonding that occurs at the _____ _____.
|
central atom
|
|

This shape consists of a central atom with four individual electron pairs.
|
Tetrahedron
|
|

This shape consists of 3 single bonds and 1 non-bonded pair
|
Trigonal Pyramid
|
|
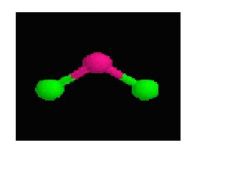
This shape consists of 2 single bonds and 2 non-bonded pairs.
|
Bent 109 degrees
|
|

This shape consists of 1 single bond and 3 non-bonded pairs.
|
Linear
|
|
This shape consists of 1 double bond and 2 single bonds.
|
Trigonal Plane
|
|

This shape consists of 1 double bond, 1 single bond, and 1 non-bonded pair.
|
Bent 120 degrees
|
|

This shape consists of 1 double bond and 2 non-bonded pairs.
|
Linear
|
|

This shape consists of 1 triple bond and 1 single bond.
|
Linear
|
|

This shape consists of 1 triple bond and 1 non-bonded pair.
|
Linear
|
|

This shape consists of 2 double bonds.
|
Linear
|
|
|
Are tetrahedrons polar, nonpolar, or both?
|
If all of the outer atoms are the same, then they are nonpolar. If not, then they are polar.
|
|
|
Are trigonal pyramids polar, nonpolar, or both?
|
polar
|
|
|
Are bent 109 degree shapes polar, nonpolar, or both?
|
polar
|
|
|
Are trigonal planes polar, nonpolar, or both?
|
If all of the outer atoms are the same, then the shape will be nonpolar. If not, then they are polar.
|
|
|
Are bent 120 degree shapes polar, nonpolar, or both?
|
polar
|
|
|
Are linear molecules polar, nonpolar, or both?
|
If all of the atoms are the same, then they are nonpolar. If not, then they are polar.
|

