![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Kekulé Model |
> Alternating single and double bonds. > Constantly flipping between two isomers.
|
|
|
Delocalised Model |
> P-orbitals of all 6 carbon atoms overlap to create pi system > 2 ring shaped clouds of electrons one above one below plane of carbon atoms. |
|
|
What evidence is there to disprove Kekulés model of benzene? |
1) Benzene not very reactive, doesn't decolourise bromine water liked you'd expect. 2) Lengths of carbon bonds all the same, in between single and double bonds. 3) Hydrogenation enthalpy less exothermic than expected compared to cyclohexene. |
|
|
What is the mechanism for nitration of benzene |
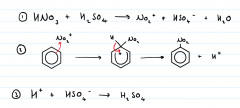
|
|
|
What type of reaction does benzene usually undergo? |
Electrophilic Substitution |
|
|
What conditions are needed to react benzene with Bromine water? |
Hot Benzene + UV Light |
|
|
Why doesn't benzene undergo electrophilic addition reactions like alkenes? |
> In alkenes the pi bond is area of high electron density. > In pi system negative charge spread out so less attraction to electrophiles. |
|
|
Give a general electrophilic substitution reaction mechanism? Use 'E' to represent an electrophile |

|
|
|
Give an example of a halogen carrier? |
AlCl3 , AlBr3 |
|
|
Why are halogen carriers used? |
Used to polarise compounds to make stronger electrophiles. |
|
|
How do halogen carriers improve electrophiles? |
1) Accept lone pair from halogen atom on electrophile. 2) Lone pair pulled away, increased polarisation and sometimes carbocation formed. |
|
|
How do halogen carriers help with halogenation of benzene? |
Polarise halogen, making one halogen an electrophile. |
|
|
Give the mechanism for halogenation of benzene? Use Bromine |

|
|
|
How do you carry out friedel-crafts reactions? |
Reflux benzene with halogen carrier and haloalkane or acyl chloride. |
|
|
What is alkylation? |
Putting an alkyl group onto benzene ring. |
|
|
What is acylation? |
Substituting H atom for acyl group. |
|
|
What is produced from acylation? |
Phenyl ketones |
|
|
During nitration of benzene what happens if temperature goes above 50 degrees? |
Further substitution reactions may occur giving dinitrobenzene. |
|
|
What catalyst is used for nitration of benzene and why is it used? |
> Concentrated sulfuric acid > Helps make nitronium ion, electrophile. |
|
|
What is the formula for phenol? |
C6H5OH |
|
|
When does it become an alcohol not a phenol? |
If -OH group attached to carbon side chain |
|
|
What's more reactive, phenol or benzene? |
Phenol as the -OH group makes it more likely to undergo electrophilic substitution |
|
|
Why is Phenol more reactive than Benzene? |
> 1 lone pair of electrons in p orbital of oxygen overlaps with delocalised ring. Lone pair then partially delocalised in pi system. > Increases electron density of ring, more likely to be attracted by electrophiles. |
|
|
What happens when phenol is dissolved in water? |
It partially dissociates to give a phenoxide ion and a proton. |
|
|
How can you distinguish between phenols and carboxylic acid? |
Add sodium carbonate, only carboxylic acid reacts and produces carbon dioxide released as gas. |
|
|
What are the products when phenol is reacted with sodium hydroxide? |
Sodium phenoxide + Water |
|
|
> What happens when you shake phenol with bromine water? > What product forms? |
> It decolourises as more reactive than benzene > 2,4,6-tribromophenol > -OH group electron donating directs to these carbons |
|
|
Is nitrating phenol easier or harder than nitrating benzene? What conditions are needed? |
> Easier > Dilute nitric acid, room temperature |
|
|
What isomers are formed? Other products? |
2-nitrophenol and 4-nitrophenol > Water is other product |
|
|
What is the difference between substitution reactions and addition-elimination reactions? |
> Substitution happens in 1 step > Addition-elimination occurs in 2 distinct steps forming an intermediate molecule |

