![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
86 Cards in this Set
- Front
- Back
|
Hydrocarbons |
* Compounds that only contain carbon and hydrogen atoms |
|
|
Alkanes |
* Hydrocarbons |
|
|
Alkenes |
* Hydrocarbon |
|
|
Alkynes |
* Hydrocarbon |
|
|
Aromatic Compounds |
* Hydrocarbon |
|
|
Saturated Compounds |
* Molecules that contain only single bonds |
|
|
Unsaturated Compounds |
* Compounds with multiple bonds
|
|
|
Methane |
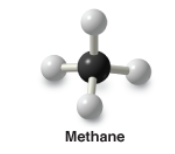
* The simplest alkane |
|
|
Ethene |
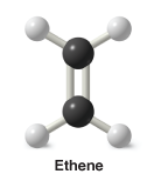
* Alkene |
|
|
Ethyne |
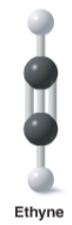
* Alkene |
|
|
Benzene |
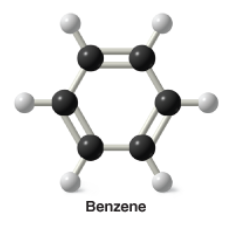
* Aromatic compounds |
|
|
Kekule Structure |
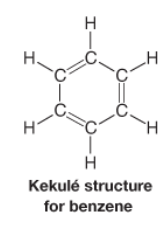
* Six membered ring with alternating double and single bonds |
|
|
Molecular Orbital Explanation for Benzene Ring |
* Carbon atoms are sp2 hybridized |
|
|
Polar Covalent Bond |
* Electronegativity differences exist between 2 covalently bonded atoms |
|
|
Dipole Moment (mu) |
* Product of the charge in electrostatic units (esu) and the distance that separates them in cm |
|
|
Debye (D) |
* 1e-18 esu-cm |
|
|
Functional Groups |
* The particular group of atoms in a molecule that primarily determines how the molecule reacts - Alkanes do not have a functional group |
|
|
Heteroatoms |
* Atoms that form covalent bonds and have unshared electron pairs |
|
|
Map of Electrostatic Potential (MEP) |
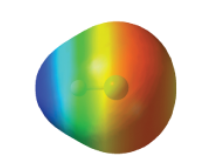
* More negative surface colored red |
|
|
Polar Molecule |
Molecule with a dipole moment |
|
|
Alkyl Groups |
* The designation given to afragment of a molecule hypothetically derived from an alkane by removing a H atom |
|
|
Methyl Group |

- Alkyl Group |
|
|
Ethyl Group |
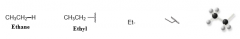
- Alkyl Group |
|
|
Propyl Group |

- Alkyl Group |
|
|
Butyl Group |

- Alkyl Group |
|
|
Isopropyl Group |
- Removal of the hydrogen atom from the middle carbon of propane - Alkyl Group - CH3CHCH3 |
|
|
The symbol R |
* General symbol to represent any alkyl group |
|
|
R-H |
General form of alkane |
|
|
Phenyl Group |
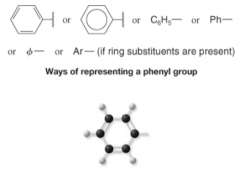
- When benzene ring is attached to some other group of atoms in a molecule - Alkyl Group |
|
|
Benzyl Group |
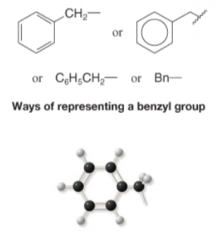
- Combination of a phenyl group and a methylene group - Alkyl Group |
|
|
Methylene group |
-CH2- |
|
|
Alkyl Halides (haloalkanes) |
* Compounds in which a halogen atom replaces a hydrogen atom of an alkane |
|
|
Primary Alkyl Halide |
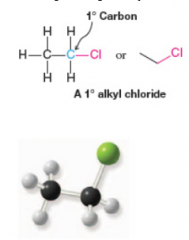
* Attached to primary carbon atom
|
|
|
Secondary Alkyl Halide |
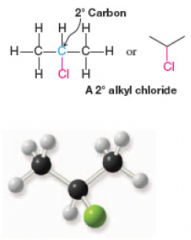
* Attached to secondary carbon atom
|
|
|
Tertiary Alkyl Halide |
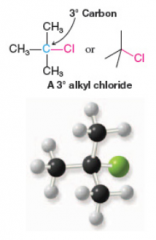
* Attached to tertiary carbon atom |
|
|
Primary Carbon Atom |
Attached to only one other carbon |
|
|
Tertiary Carbon Atom |
Attached to three other carbon atoms |
|
|
Alkenyl Halide |
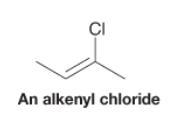
- Compound with a halogen atom bonded to an alkene carbon |
|
|
Aryl Halide |
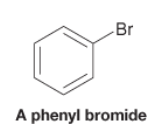
- Compound with a halogen atom bonded to an aromatic ring |
|
|
Methyl Alcohol (Methanol) |
* CH3OH
* Simplest alcohol |
|
|
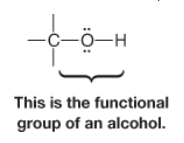
Alcohol |
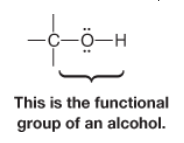
* Characterized by an hydroxyl group (-OH) attached to an sp3 hybridized carbon atom |
|
|
Ethyl Alcohol (ethanol) |
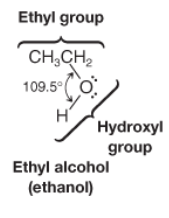
* CH3CH2OH
|
|
|
Phenol |
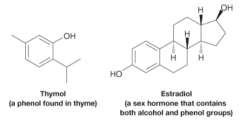
* Hydroxyl group is bonded to a benzene ring |
|
|
Ether |
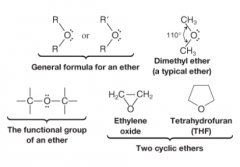
* General Formula R-O-R', where R' is a different alkyl (or phenyl) group than R
* Can be thought of as water in which both H atoms have been replaced by alkyl groups * Bond angle at O is slightly larger than water |
|
|
Amines |
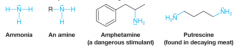
* Can be considered an organic derivative to ammonia |
|
|
Primary Amine |
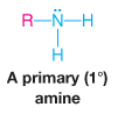
|
|
|
Secondary Amine |
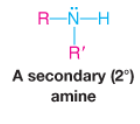
|
|
|
Tertiary Amine |
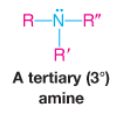
|
|
|
Bonding Order for MO Theory (Ch. 1) |
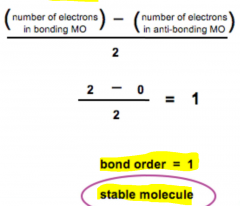
- IF 0, two atoms are not bonded 1 = single bond 2 = double bond 3 = triple bond - |
|
|
Carbonyl group |
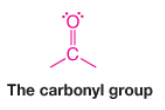
Group in which a carbon atom has a double bond to O |
|
|
Aldehyde |
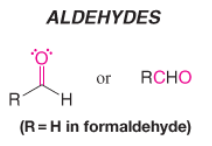
* Contains carbonyl group bonded to one H and one C |
|
|
Ketone |
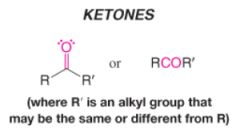
* Contains a carbonyl group bonded to 2 carbon atoms |
|
|
Amides |
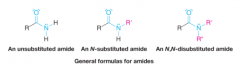
* Contain a carbonyl group bonded to a N atom bearing H and or alkyl groups |
|
|
Carboxyl group |
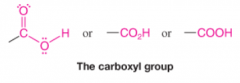
* Carbonyl and hydroxyl groups
|
|
|
Carboxylic Acids |
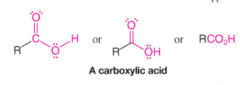
* Contain a carbonyl group bonded to a hydroxyl group
|
|
|
Esters |
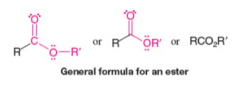
* Contain a carbonyl group bonded to an alkoxyl (-OR) group
* Can be made from carboxylic acid and an alcohol via acid catalyzed loss of water molecule |
|
|
Formic Acid |
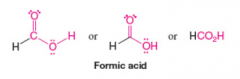
Carboxylic Acid |
|
|
Acetic Acid |
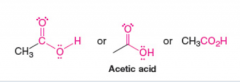
Carboxylic Acid |
|
|
Benzoic Acid |
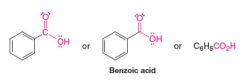
Carboxylic Acid |
|
|
Alkoxyl group |
* -OR group
|
|
|
Ethyl Acetate |
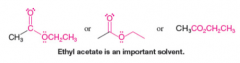
* Important solvent |
|
|
Nitriles |
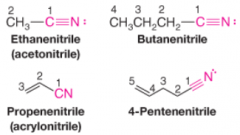
* R group bonded to a CN group
* C and N are sp hybridized * Named by adding -nitrile to the name of the corresponding hydrocarbon * C of CN group is assigned number 1 |
|
|
Cyclic Nitriles |
Add suffix carbonitrile to name of the ring system |
|
|
Melting Point |
* the temperature at which an equilibrium exists between the well-ordered crystalline state and the more random liquid state |
|
|
Ion-ion forces |
* Strong electrostatic forces of attraction between ions of opposite charges. |
|
|
Boiling Point |
* The temperature at which the vapor pressure of a liquid is equal to the pressure above the surface of the liquid. |
|
|
Van der Waals forces |
* Intermolecular forces
* Electrical in nature * Dipole-Dipole, Hydrogen Bonds, and dispersion forces |
|
|
Dipole-dipole forces |
* Molecules that are not fully ionic but have a permanent dipole moment due to nonuniform distribution of bonding electrons |
|
|
Dispersion Forces (London Forces) |
* Results from temporary dipole due to instant nonuniform distribution of electrons in a non-polar molecule
* Polarizability of electrons in atoms involved increases magnitude * Larger surface area of molecule increases magnitude |
|
|
Dispersion Forces - polarizability |
* How easily electrons respond to a changing electric field |
|
|
Dispersion forces - surface area |
* Longer, flatter cylindrical molecules have larger surface area
* Branched molecules have less |
|
|
Solubility |
* The extent to which a given solute dissolves in a given solvent, usually expressed as a weight per unit volume
|
|
|
Ion-dipole forces |
* The interaction of an ion with a permanent dipole. Such interactions (resulting in solvation) occur between ions and the molecules of polar solvents.
|
|
|
General Rules of Solubility |
* Polar and ionic solids are usually soluble in polar solvents
* Polar liquids are usually miscible. * Nonpolar solids are usually soluble in nonpolar solvents. * Nonpolar liquids are usually miscible. * Polar and nonpolar liquids, like oil and water, are usually not soluble to large extents. |
|
|
Guidelines for Water Solubility |
* For compounds containing one hydrophilic group- thus capable of forming H bonds |
|
|
Hydrogen Bonds |
* Strong dipole-dipole interactions between H atoms bonded to strongly electronegative atoms (O, N, F) and nonbonding electron pairs on other such electronegative atoms |
|
|
Infrared Spectroscopy |
* A type of optical spectroscopy that measures the absorption of infrared radiation. Infrared spectroscopy provides structural information about functional groups present in the compound being analyzed. |
|
|
Wavenumber (`V) |
* Number of waves per cm (cm-1) |
|
|
IR Spectroscopy Vibrations |
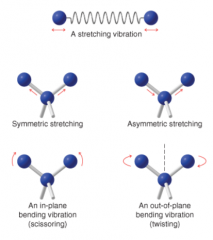
* Light atoms vibrate at higher freq than large atoms |
|
|
IR Spectra C-C Bonds |
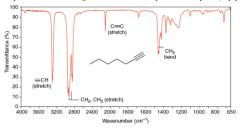
* Single bonds give rise to weak peaks that are usually of little use in assigning structures
* Carbon-carbon double bonds give absorption peaks in the 1620-1680 (1/cm) region * carbon-carbon triple bonds give absorption peaks between 2100 and 2260 (1/cm) * If absent, the double or triple bond is symmetrically substituted (no dipole moment change) * The stretchings of the carbon-carbon bonds of benzene rings usually give a set of characteristic sharp peaks in the 1450-1600 (1/cm) region |
|
|
IR Spectra Carbonyl Groups |
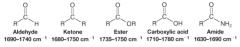
* carbon-oxygen double-bond stretching frequency of carbonyl groups gives a strong peak between 1630 and 1780 (1/cm)
* The exact location of the absorption depends on whether it arises from an aldehyde, ketone, ester, and so forth |
|
|
IR Spectra Hydroxyl Groups |
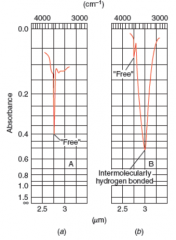
* absorption of an alcohol or phenol O─H group is in the 3200-3550 (1/cm) range, and most often it is broad
* Without IM hydrogen bonds, absorption has a much sharper peak at 3590-3650 (1/cm) |
|
|
IR Spectra Carboxylic Acids |
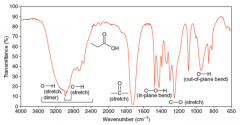
* The hydroxyl absorption of a carboxylic acid is often very broad, extending from 3600 to 2500 (1/cm)
* If both carbonyl and hydroxyl absorptions are present, good evidence for presence of a carboxylic acid group, but groups could be isolated in molecule |
|
|
IR Spectra Amines |
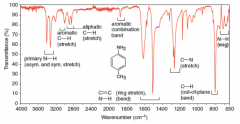
* Primary (1°) and secondary (2°) amines give absorptions of moderate strength in the 3300-3500 (1/cm) region
* Primary amines exhibit two peaks in this region due to symmetric and asymmetric stretching of the two N─H bonds. * Secondary amines exhibit a single peak. * Tertiary amines show no N─H absorption because they have no such bond. * A basic pH is evidence for any class of amine |
|
|
IR Spectra C-H Bonds |
* C-H stretching peaks of H atoms attached with an sp orbital is about 3300 (1/cm) |
|
|
Resonance Structures - Use of Curved Arrows |
- show the direction of electron flow in a reaction mechanism - point from the source of an electron pair to the atom receiving the pair - always show the flow of electrons from a site of higher electron density to a site of lower electron density - never show the movement of atoms. atoms are assumed to follow the flow of electrons |

