![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
|
Element |
A substance that can't be broken down or converted to other substances by ordinary means |
|
|
Atoms |
Smallest possible piece of an element |
|
|
Isotope |
Atoms of the same element have different number of neutrons. Most are stable. |
|
|
Ion |
Atom that has gained or lost an electronic |
|
|
What makes a molecule? |
2 atoms |
|
|
Proton |
Positively charged particles |
|
|
Neutron |
Neutral particles |
|
|
Electrons |
Negatively charged particles |
|
|
Atomic number |
Number of protons in the nucleus of an atom |
|
|
Atomic mass |
Total mass of all the protons and neutrons |
|
|
Molecules |

|
|
|
Compound |

|
|

|

|
|
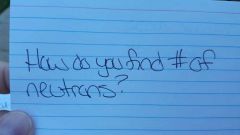
|

|
|

|

|
|
|
What are electron shells? |

|
|

|
Higher |
|

|

|
|

|

|
|
|
Polar covalent bonds |

|
|
|
Nonpolar covalent bonds |

|
|
|
Hydrogen bonding |

|
|
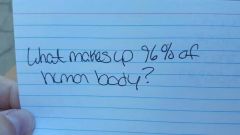
|

|
|

|

|
|

|

|
|

|

|
|

|

|
|
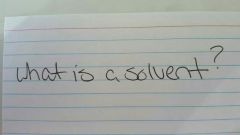
|

|
|

|

|
|

|

|
|

|

|
|

|

|
|

|

|
|
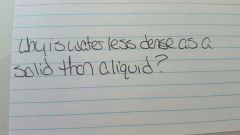
|

|
|
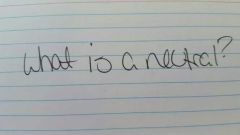
|

|
|

|

|
|
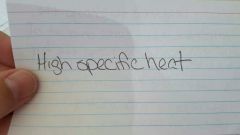
|

|
|
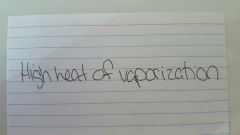
|

|
|
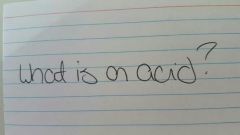
|

|
|
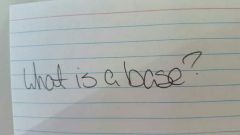
|

|

