![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
Matter and Atomic Theory |
The basis of chemical reactions in cells is based on the instability of atoms, involving charged protons and uncharged neutrons in the atomic nucleus, and the charged electrons in electron shells. |
|
|
Isotopes and Ions |
Variation in the number of neutrons generates isotopes of an element whereas variation in the number of electrons produces ions. |
|
|
Electron Placement |
The chemical properties and reactivity (bonding) of an element depend on the number of electrons in the outermost shell. |
|
|
Ionic Bonds |
form salts and are important in protein structure. |
|
|
Covalent Bonds |
form molecules through a sharing of electron pairs and are the predominant bonds in biologically important compounds. Such pairing of electrons can be equal (nonpolar covalent bonds) or unequal (polar covalent bonds). |
|
|
Hydrogen Bonds |
result from polar covalent bonds between oxygen (or nitrogen) and hydrogen. These bonds are keys to the properties of water, protein structure, and DNA structure. |
|
|
Chemical Reactions |
Chemical bonding is explained by molecules and compounds interacting with one another through chemical reactions. This brings us full circle because the reactions are the result of atoms filling their outermost electron shells to form a stable configuration. |
|
|
The properties of water |
The whole chemical basis of life is based on water as a solvent as well as its other unique properties. |
|
|
The concept and application of pH |
Biological molecules and organisms in general are sensitive to varying acidic or basic conditions and this is made perfectly clear with microorganisms. Neutrality is the key to survival although microbes can adapt to extremes, especially on the acidic side of the pH scale. |
|
|
Buffers |
Although the environment may vary in acidity, the organism’s ability to survive depends on its ability to maintain an internal pH near 7. This means organisms must have buffer systems that can donate or eliminate hydrogen ions to prevent internal pH fluctuations |
|
|
The importance of functional groups |
Relatively stable chemical compounds interact through functional groups present on the monomers of carbohydrate, lipids, nucleic acids, and proteins. |
|
|
The Carbohydrates |
The monosaccharides play roles in energy metabolism, as energy storage compounds (polysaccharides), and as structural cell wall polymers in bacteria (linked disaccharide units), archaea, algae, and fungi. |
|
|
Lipids |
Although some microbes store lipids for energy, the phospholipids are critical to all organisms in the structure of cell membranes |
|
|
Nucleic Acids |
DNA and RNA are critical genetic information and regulatory compounds in all organisms and viruses. The ability of genes to produce proteins is the key to cellular metabolism, growth, and reproduction. |
|
|
Proteins |
the workhorses in cells, carrying out a myriad of functions. This demands that each cellular reaction have a specific protein (enzyme) with a specific shape to carry out the reaction or a shape that allows it to carry out its functional or structural role. To come full circle, changes in pH can denature proteins, causing a loss of function that can cripple or even kill a cell or organism. |
|
|
Acid |
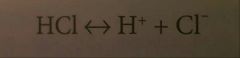
A chemical substance that donates H+ to a solution, increasing the H ion concentration |
|
|
@ggffvbw |
Wvjsjwjuwjv |
|
|
Gyy |
Ggy |

