![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
71 Cards in this Set
- Front
- Back
|
Matter
|
All such tangible materials that occupy space and have mass.
|
|
|
Atoms
|
Tiny particle that cannot be subdivided into smaller substances without losing its properties. Building blocks.
|
|
|
Elements vs isotopes
|
Elements are a varied combinations of subatomic particles (electrons, protons, neutrons) form unique type of atoms-pure, structural and predictable behavior Isotopes are unstable, various forms of the same element that differ in the number of neutrons and have different mass numbers. |
|
|
Protons
|
Subatomic particles which are positively charged |
|
|
Electrons
|
Subatomic particles which are negatively charged.
|
|
|
Neutrons
|
subatomic particles which have no charge, neutral. |
|
|
Element
|
When subatomic particles come together in specific, varied combinations, unique types of atoms structural and predictable chemical behavior.
|
|
|
Atomic Number
|
Each element is assigned a atomic number (AN)based on the number of protons.
|
|
|
Mass Number
|
Useful measurement equal to the number of protons and neutrons. *Subtract the mass number and the atomic number to determine the number of neutrons . |
|
|
Isotopes
|
Variant forms of the same element that differ in the number of neutrons and thus have different mass numbers.
|
|
|
Atomic Weight
|
An important measurement of a element that gives the average mass numbers of all isotopic forms.
|
|
|
Biological Important Elements |
the following elements comprise 97% of the dry mass in living cells. 47% Carbon 30% Oxygen 9% Hydrogen 8% Nitrogen 3% Phosphorous 2% Sulfur, Potassium, Calcium, Sodium, Chlorine, Magnesium. 1% others. |
|
|
Abbreviations of Elements
|
Ca Calcium I-131 Iodine* C Carbon Fe Iron C-14 Carbon* Mg Magnesium Cl Chlorine Mn Magnese Co Cobalt N Nitrogen Cu Copper O Oxygen H Hydrogen P Phosphorous H-3 Hydrogen* P-32 Phosphorous* K Potassium Na Sodium S Sulfur Zn Zinc I Iodine |
|
|
Isotopes of the same element
|
If two atoms have the same number of protons and electrons but different numbers of neutrons.
|
|
|
Electron Orbital Shells
|
The number of electrons in the outer shells is an indicator of how reactive the atom will be.
|
|
|
Molecule
|
Chemical substance that results from the combination of two or more atoms. ex. H20-cannot be accompanied. |
|
|
Compound
|
Molecules that are combinations of two or more different elements. Can be a molecule. |
|
|
Formula/Mass weight
|
Sum of all the atomic masses of the atoms a molecule contains. |
|
|
Chemical bonds
|
when 2 or more atoms share, donate, or accept electrons to form molecules and compounds. 3 basic types: 1) Covalent 2) Ionic 3) Hydrogen |
|
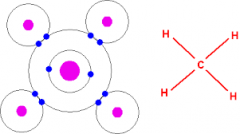
Covalent bonds |
Electrons are shared among atoms. * Molecules are stable when outer shells are full. Example: Hydrogen bond, Methane gas. |
|
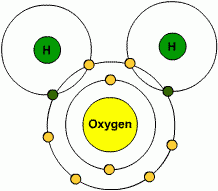
Polar covalent bonds
|
Unequal sharing or distribution of charges- it has positive and negative poles. ex. H20. the oxygen atom is larger and has more protons than the hydrogen atoms, it will have a stronger attraction than the hydrogen atoms. Electrons spend more time with O2. |
|
|
Nonpolar covalent bonds
|
Electrons are shared equally between two atoms. This balanced distribution, no part of the molecule has a greater attraction for the electrons makes this molecule electrically neutral. |
|
|
Polarity |
When covalent bonds occur between two atoms with different atomic masses, the electrons tend to spend more time toward the larger atom (more electronegativity)
|
|
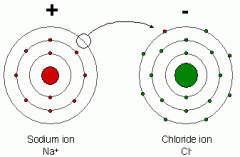
Ionic bonds
|
Electrons are transferred to one atom forming positively charged cations, negatively charged anions. If one atom is so charged that is pulls an electron from the other atom. NaCl |
|
|
Hydrogen bonds
|
Weak bonds between water molecules. Formed when more electronegative oxygen attracts the electrons.
|
|
|
Oxidation
|
The loss of electrons such as sodium loses its electron to chlorine and becomes more positively charged ion - cation |
|
|
Reducing agent (Na)
|
When sodium donates a electron to chlorine it becomes the Reducing agent because it reduced another atom. |
|
|
Reduction
|
The gaining of electrons and coverts to a anion. Like chlorine does when it receives a electron from sodium. |
|
|
Oxidizing agent
|
An atom that receives a extra electron oxidizes another molecule making more positively charged. ex. chlorine in NaCl |
|
|
Redox reactions
|
Oxidation-reduction reaction. When electrons are transferred from one atom or molecule to another.
|
|
|
Reactants
|
Molecules involved and starting a reaction and that are changed by the reaction.
|
|
|
Products
|
Substances left by the reactions |
|
|
Synthesis reaction |
S + O2 > SO2 |
|
|
Decomposition Reaction
|
The bonds on a single reactant molecule are broken to release two or more products 2H2O2 > 2H2O + O2 |
|
|
Exchange Reaction |
HCL + NaOH > NaCL + H2O |
|
|
Solution |
a mixture of one or more substances called solutes in solvent. |
|
|
Solutes |
one or more substances uniformly dispersed in a dissolving medium.
|
|
|
Solvent
|
a medium. |
|
|
Hydrophilic molecules |
dissolve in water |
|
|
Hydrophobic molecules
|
repel water |
|
|
Amphipathic molecules
|
example: soap, or phospholipid. |
|
|
Acidity (acidic)
|
PH below 7. |
|
|
Alkalinity (Basic)
|
When a component releases excess hydroxide ions . Anything above pH of 7. |
|
|
pH scale
|
example: pH 2 contains [0.01 moles H+/L] pH 2 has an [H+] of 10-2 |
|
|
Organic chemicals
|
|
|
|
Carbon (backbone or organic compounds) |
*can form single, double, or triple covalent bonds. *can form linear, branched, or ringed molecules. |
|
|
Inorganic |
ex: H2O, O2, NaCl, Mg3(PO4)2 |
|
|
Functional group |
Accessory molecules that bind to organic compounds. confer unique reactive properties on the whole molecule. Hydroxyl, Carboxyl, Amino, Ester, Sulfhydryl, carbonyl, phosphate. |
|
|
Macromolecules
|
Biochemicals are organic compounds produced by living things. Large compounds assembled from smaller subunits. |
|
|
Monomer
|
a repeating unit or subunits except lipids are formed by polymerization. |
|
|
Polymer
|
a chain of monomers bound into chains of various lengths. |
|
|
4 basic macromolecules
|
2) lipids 3) proteins 4) nucleic acids. |
|
|
Carbohydrates |
Monosaccharides - 3-7 carbon sugars. Glucose, fructose Disaccharides- two monosaccharides. Maltose, Lactose, Sucrose. Polysaccharides- chain of monosaccharides. starch, cellulose, |
|
|
Lipids
|
Triglycerides fatty acids+glycerol - fats, oils, storage. Phospholipids fatty acids + glycerol + glycerol. Membranes waxes fatty acids + alcohols - mycolic acid steroids ringed structures - cholesterol, ergosterol |
|
|
Proteins
|
amino acids in chain bound by peptide bonds - enzymes. |
|
|
Nucleic acids
|
Deoxyribonucleic acid (DNA) - |
|
|
Carbohydrates
|
-Monosaccharide: 3-7 carbons -Disaccharide: two monosaccharides -Polysaccharide: five or more monosaccharides. Functions: cell structure, adhesion, metabolism and energy. |
|
|
Carbohydrates
|
|
|
|
Dehydration synthesis
|
loss of water in a polymerization reaction. Polymerization forms polymers. |
|
|
Lipids |
-long or complex, phospholipid in membranes, steroids like cholesterol. Functions: Triglycerides-energy storage. Phospholipid- major cell membrane component Steroids- cell membrane component. |
|
|
Triglycerides
|
*triglycerides are used for energy storage- no double bonds. * could be saturated or unsaturated saturated-no double bonds unsaturated-a lot of double bonds. |
|
|
Phospholipid
|
unipolar, phosphate group. glycerol with 2 fatty acids and a phosphate group *bilayers of phospholipids form membranes. |
|
|
Steroids
|
cell membrane component- cholesterol. |
|
|
Proteins
|
Monomer- amino acids- 20 different naturally occurring forms make up protein, backbone carbon. Amino & carboxyl. polymer-peptide, polypeptide, protein. Functions: support enzymes, transport, defense and movement. |
|
|
Proteins
|
* fold into very specific 3-D shapes * Functions: support, enzymes, transport, defense, movement. |
|
|
Amino Acids
|
polymer. -are attached through peptide bonds to form proteins. |
|
|
Nucleic Acids
|
* DNA and RNA * Nucleotide monomer * DNA- deoxyribonucleic acid- A,T,C,G double helix, function-heredity material - deoxyribose. * RNA- ribonucleic acid- A,U,C,G-nitrogen bases function- organize protein synthesis, single strand. - ribose |
|
|
Nucleotide Components (heredity material) |
*DNA Nucleotides Doxyribose C,G,A, or T Double helix * RNA Nucleotides: Ribose C,G,A or U single strand |
|
|
Double Helix of DNA
|
*each stand is copied. * replication is guided by base pairing. * End result is two separate double strands. |
|
|
ATP
|
* adenosine triphosphate -Nucleotide-adenine, ribose, three phospates
* Function- transfer and storage of energy. |
|
|
|
|

