![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
Electronegativity |

Is the ability of an atom to attract shared pairs of electrons to it. So, the more electronegative the atom is, the more it will attract electrons.
|
|
|
Nonpolar Covalent Bond |
Is a covalent bond in which the shared electrons are shared equally |
|
|
Polar Covalent Bond |
Is a covalent bond in which the shared electrons are more attracted to one of the atoms than the other, thereby forming a polar molecule The shared electrons are more likely to be near the atom whose electronegativity is higher |
|
|
Ionic Character |
Refers to the percentage of difference between the electronegativity of two covalently bonded atoms The greater the electronegativity the higher the ionic character |
|
|
Valence Shell Electron Pair Repulsion (VSEPR Theory) |
Helps predict the shape of any molecule given its Lewis dot structure. |
|
|
Intermolecular Forces |
Are the forces that exist between molecules Effects
|
|
|
Hydrogen Bond |
Is an attraction between a slightly positive hydrogen on one molecule and a slightly negative atom on another molecule It is a dipole-dipole attraction Strongest among intermolecular forces |
|
|
Dipole-Dipole Force |
Is when the positive side of a polar molecule attracts the negative side of another polar molecule. Molecules need to be close together |
|
|
Ion-Dipole Force |
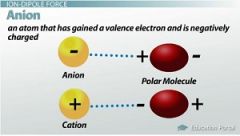
Is a force between an ion and a polar molecule |
|
|
London Dispersion Force (Van der Walls) |
Is the weak intermolecular force that results from the motion of electrons that creates temporary dipoles in molecules.
A London dispersion force works because of the movement of electrons. As you can imagine, the more electrons in the atoms, the stronger the force London dispersion force increases with increasing atomic mass. |
|
|
Sigma Bond |
Is when two orbitals directly overlap but there is only one bonding interaction |
|
|
Pi Bond |
Is weaker than the sigma bond. This overlap occurs when two orbitals overlap and there are two bonding interactions. It looks like two dumbbells put side-by-side and overlapped. |
|
|
Hybridization |
Is the mixing of two or more atomic orbitals to form new orbitals that describe the covalent bonding in molecules The number of hybrid orbitals is the same as the number of atomic orbitals that participate in the process. |
|
|
Molecular Orbital Theory |
Describes how the orbital shapes combine when atoms combine into molecules. It makes the assumption that electrons don't belong to any individual atom, but are under the influence of the entire molecule and belong to the entire molecule as a whole. |
|
|
Metal Bonds |
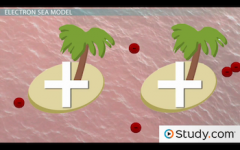
Most metals have very few electrons in their outermost energy shells, and some have vacant outer electron orbitals. What this means for the metal is that its valence electrons are decentralized and free to move around. Metals do not form; ionic, compound, or molecular bonds |
|
|
Properties of Metal |
Metal is shiny because it reflects incoming light photons. It is malleable because the structure and uniform bonding in all directions of the metal allow the atoms to slide past each other without breaking. Metal conducts electricity well because all of the mobile electrons are free to move towards any attraction. |
|
|
Organic Macromolecules Include Four Classes |
|
|
|
Proteins |
Are formed through a condensation reaction between amino acid monomers A condensation reaction is when two molecules combine to form a larger molecule but lose a small molecule, kind of like a waste product |
|
|
Lipids |
Lipids are hydrophobic, meaning they hate water, and contain a lot of C-H bonds |
|
|
Carbohydrates |
These polysaccharides are made up of monomer monosaccharides, or simple sugars, like fructose and glucose. Carbohydrates contain only C, H and O. |
|
|
Nucleic Acids |
Are made up of nucleotide monomers A nucleotide has three parts - a phosphate group, a five-carbon sugar and a nitrogen base. |
|
|
Saturated Hydrocarbon |
Are hydrocarbons that contain no rings and contain only single bonds between the different atoms Makes up what we call "natural gas" |
|
|
Saturation Formula |
Saturated hydrocarbons have 2N + 2 hydrogen atoms, where N is the number of carbon atoms in the molecule |
|
|
Alkane |
A hydrocarbon that contains no double bonds, or hydrocarbon containing only single bonds |
|
|
Alkene |
Is a hydrocarbon that contains a carbon-carbon double bond |
|
|
Alkynes |
Hydrocarbons that contain carbon-carbon triple bonds |
|
|
Isomers |
Molecules that have the same chemical formula but different structures |
|
|
Aromatic Hydrocarbons |
These hydrocarbons that contain rings consisting of alternating single and double carbon-carbon bonds were initially called this because of their strong smells |
|
|
Functional Groups |
Atoms or groups of atoms that are responsible for particular chemical properties and reactions of organic compounds.
|
|
|
Esters |
Esters are derivatives of carboxylic acids. The -OH of the carboxyl group gets replaced by an -OR from an ether R-COOR |
|
|
Carboxylic Acids |
Is a compound with a carboxyl group. A carboxyl group is -COOH RCOOH |
|
|
Ethers |
Is an organic compound where oxygen is bonded to two carbon atoms ROR |
|
|
Aldehydes |
Is a compound in which the carbon of a carbonyl group shares bonds with two other carbons. A carbonyl group is an oxygen double-bonded to a carbon RCHO |
|
|
Ketones |
Is a compound in which the carbon of a carbonyl group shares bonds with two other carbons. A carbonyl group is an oxygen double-bonded to a carbon RCOR |
|
|
Alkyl Halides |
An alkyl halide is an organic compound where a halogen atom - F, Cl, Br or I (fluorine, chlorine, bromine or iodine) - takes the place of one or more hydrogens in a hydrocarbon RX |
|
|
Alcohols |
Is an organic compound that contains one or more hydroxyl groups ROH |

