![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
Molecular Formula |
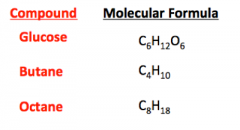
Tells us the actual number of the different elements in one molecule of a compound |
|
|
Empirical Formula |

Is defined as the simplest ratio of whole numbers of elements that make up a compound, and this type of formula is derived from experimental data. |
|
|
Structural Formula |
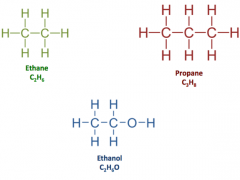
Shows both the actual number of atoms of elements in a compound, how the atoms are arranged as well as which atoms are bonded to one another |
|
|
Condensed Structural Forumula |
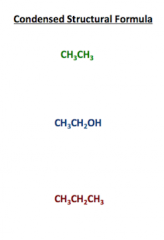
Shows the position of atoms in relation to one another without showing their bonds. |
|
|
Octet Rule |
Atoms like to have full outer shells of only eight electrons. |
|
|
Lewis dot structure |

A quick and easy diagram that shows the valence electrons in an element |
|
|
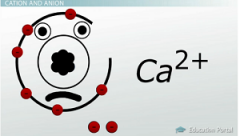
Atoms can gain or lose more than one electron at a time. If they do, they are written with the superscript of what they have gained or lost. Ca2+, for instance, has lost two electrons. |
|
|
When Atom Lose/Gain Electrons |
If they have less than three valence electrons, it is easier to lose them and become a positively charged ion. If they have more than four valence electrons, it is easier to gain electrons and become negatively charged.
|
|
|
Ionic Compounds |
Is a compound held together by ionic bonds Remember that an ionic bond is formed through the transfer of electrons. These compounds are usually formed between +metals and -non-metals. The ratio of cations to anions is always in a way that there is no net charge. |
|
|
Lattice Energy |
The way that the strength of ionic compounds is measured. It is the energy released when one mole of an ionic compound is formed. This means when the individual ions of the compound come together to form the crystal lattice, they need less energy to stay together, so they release it, and the energy released is called the lattice energy The bond force between ions of opposite charge is strongest when the ions are small. The bond is also stronger as the charge on the ions get larger So, the force of the bond between a +1 cation and a -1 anion isn't as strong as the force between a +3 cation and a -2 anion. |
|
|
Properties of Ionic Compounds (5) |
|
|
|
Naming Binary Ionic Compounds Rules |
A simple binary compound is just what it seems - a simple compound with two elements in it. |
|
|
Naming Ionic Compounds Containing Transition Metals Example: FeCl2, FeCl3 |
A transition metal is a metal that can use the inner shell before using the outer shell to bond. These are the elements in the middle of the periodic table - things like zinc, iron and copper. |
|
|
Naming Polyatomic Ionic Compounds Example: CIO, CIO2, CIO3, CIO4 |
Polyatomic ionic compound: is a compound made up of a polyatomic ion, which is two or more atoms bonded together, and a metal When a hydrogen ion is involved, the compound starts with either hydrogen or dihydrogen, depending on if there are one or two ions involved. |
|
|
Rules for Writing Chemical Formulas for Binary and Rolyatomic Ionic Compounds Examples Sodium oxide Iron (III) oxide Potassium phosphate iron (III) chromate |
|
|
|
Covalent Compounds |
Is made when two or more nonmetal atoms bond by sharing valence electrons = Covalent Bond |
|
|
Nonmetals |
Are types of elements that lack metallic characteristics
|
|
|
Types of Covalent Bonds Single Double Triple |
Two shared electrons are known as a single covalent bond Four shared electrons are known as a double bond Six shared electrons are known as a triple bond |
|
|
Electronegativity |
The bonded electrons between two chlorine atoms are shared evenly - each atom exerts the same pull on the shared electrons. This isn't always the case Is the ability of an atom to draw electrons to itself. If a covalent bond is made between one atom that is really electronegative and another that is not, the electrons will not be shared evenly in the bond = dipole |
|
|
Dipole |
When electrons in a covalent bond are not shared evenly between the two atoms |
|
|
Properties of Covalent Bonds |
|
|
|
Naming Simple Covalent Compounds Examples N20 SO3 N2H4 |
Dinitrogen monoxide Sulfur trioxide Dinitrogen tetrahydride |
|
|
Lewis Dot Polyatomic Ions OH- |
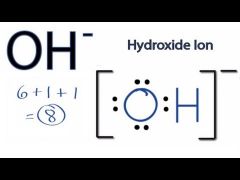
The charge on a polyatomic atom indicates the number of electrons that have been lost or gained by a molecule -- you add this to the total of valence electrons for Lewis Dot Structures |
|
|
Resonance |
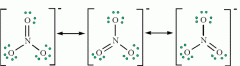
When a molecule or polyatomic ion has multiple valid Lewis dot structures Resonance structures are shown together, separated by double headed arrows. |

