![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
32 Cards in this Set
- Front
- Back
|
Homolysis
|
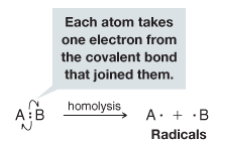
- The cleavage of a covalent bond so that each fragment departs with one of the electrons of the covalent bond that joined them. - generates radicals - Energy in the form of heat or light must be supplied to cause homolysis of covalent bonds |
|
|
Peroxides
|

- A compound with an oxygen-oxygen single bond. - undergo homolysis readily when heated, because the oxygen.oxygen bond is weak. - produces 2 alkoxyl radicals |
|
|
Halogen Molecules
|
- contain a relatively weak bond - halogens undergo homolysis readily when heated or when irradiated with light of a wavelength that can be absorbed by the halogen molecule |
|
|
Reactions of Radicals
|
- Almost all small radicals are short-lived, highly reactive species - When radicals collide with other molecules, they tend to react in a way that leads to pairing of their unpaired electron |
|
|
Hydrogen Abstraction
|
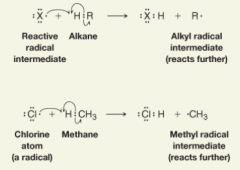
- To abstract an atom means to remove an atom by homolytic bond cleavage as the atom forms a bond with another radical - gives the halogen atom an electron (from the hydrogen atom) to pair with its unpaired electron. - the other product of this abstraction is another radical intermediate, in this case, an alkyl radical, R⋅, which goes on to react further, |
|
|
Radical Addition to a Pi Bond
|
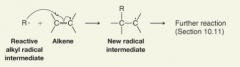
|
|
|
Homolytic Bond Dissociation Energies (DH)
|
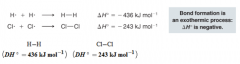
- The energies required to break covalent bonds homolytically - The order of stability of alkyl radicals is the same as for carbocations |
|
|
Radical Holgenation
|
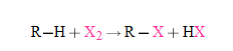
- Alkanes react with molecular halogens to produce alkyl halides - the mechanism involves species with unpaired electrons called radicals. - This reaction is not a nucleophilic substitution reaction - A halogen atom replaces one or more of the hydrogen atoms of the alkane, and the corresponding hydrogen halide is formed as a by-product - Only fluorine, chlorine, and bromine react this way with alkanes. Iodine is essentially unreactive due to unfavorable reaction energetics |
|
|
Multiple Halogen Substitution
|
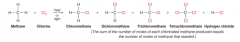
- multiple substitutions almost always occur unless we use an excess of the alkane - ix an equimolar ratio of methane and chlorine (both substances are gases at room temperature) and then either heat the mixture or irradiate it with light - At the outset, the only compounds that are present in the mixture are chlorine and methane, and the only reaction that can take place is one that produces chloromethane and hydrogen chloride - As the reaction progresses, however, the concentration of chloromethane in the mixture increases, and a second substitution reaction begins to occur. Chloromethane reacts with chlorine to produce dichloromethane - The dichloromethane produced can then react to form trichloromethane, and trichloromethane, as it accumulates in the mixture, can react with chlorine to produce tetrachloromethane. - Each time a substitution of ─Cl for ─H takes place, a molecule of H─Cl is produced. |
|
|
Chloronation
|
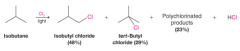
- A reaction in which one or more chlorine atoms are introduced into a molecule - Chlorination of most higher alkanes gives a mixture of isomeric monochlorinated products as well as more highly halogenated compounds. - Chlorine is relatively unselective; it does not discriminate greatly among the different types of hydrogen atoms (primary, secondary, and tertiary) in an alkane - An exception is the halogenation of an alkane (or cycloalkane) whose hydrogen atoms are all equivalent (i.e., homotopic). |
|
|
Radical Chlorination of Methane
|
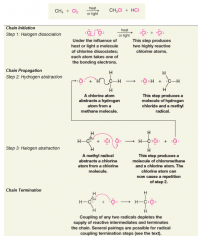
- A relatively small number of light photons permits the formation of relatively large amounts of chlorinated product - react in the dark if the gaseous mixture is heated to temperatures greater than 100 °C - chain reaction making light reactions efficient |
|
|
Chain Reaction
|
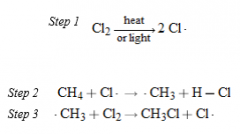
- sequential, stepwise mechanism, in which each step generates the reactive intermediate that causes the next cycle of the reaction to occur - Step 1 is called the chain-initiating step. In the chain-initiating step radicals are created. -Steps 2 and 3 are called chain-propagating steps. In chain-propagating steps one radical generates another |
|
|
Radical Halogenation of Ethane
|
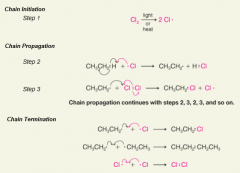
- chloronation is not selective
|
|
|
Selectivity of Bromine
|
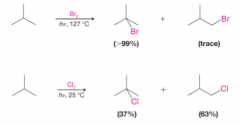
- Bromine is less reactive than chlorine toward alkanes in general but bromine is more selective in the site of attack - Bromination is selective for substitution where the most stable radical intermediate can be formed. |
|
|
Selectivity of Flourine
|
- being much more reactive than chlorine, is even less selective than chlorine - Because the energy of activation for the abstraction of any type of hydrogen by a fluorine atom is low, there is very little difference in the rate at which a 1°, 2°, or 3° hydrogen reacts with fluorine - Reactions of alkanes with fluorine give (almost) the distribution of products that we would expect if all of the hydrogens of the alkane were equally reactive. |
|
|
Geometry of Alkyl Radicals
|
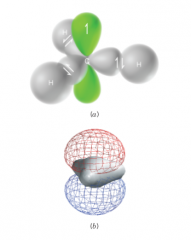
- This structure can be accommodated by an sp2-hybridized central carbon - the p orbital contains the unpaired electron |
|
|
Chloronation of Pentane
|

- When achiral molecules react to produce a compound with a single tetrahedral chirality center, the product will be a racemic form |
|
|
Stereochemistry of Chlorination at C2 of Pentane
|
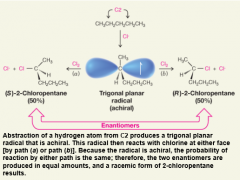
- the C2 hydrogens of pentane are enantiotopic because enantiomers are formed by reaction at each C2 hydrogen.
|
|
|
Stereochemistry of Chlorination at C3 of (s)-2-Chloropentane
|
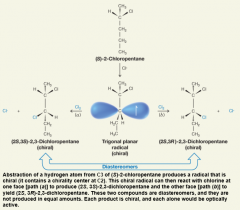
- products of the reactions are diastereomers - The two diastereomers are not produced in equal amounts because the intermediate radical itself is chiral, reactions at the two faces are not equally likely. - because the two compounds are diastereomers, they have different physical properties |
|
|
Allylic Group
|
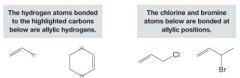
- An atom or group that is bonded to an sp3-hybridized carbon adjacent to an alkene double bond - Allylic hydrogens are especially reactive in radical substitution reactions. |
|
|
Allylic Substitution
|

- Allylic hydrogens are especially reactive in radical substitution reactions - When propene reacts with bromine or chlorine at high temperatures or under radical conditions where the concentration of the halogen is small |
|
|
Allylic Chlorination (High Temperature)
|
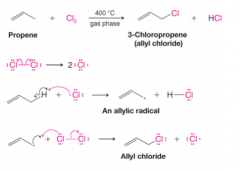
- Propene undergoes allylic chlorination when propene and chlorine react in the gas phase at . - the chain-initiating step, the chlorine molecule dissociates into chlorine atoms - In the first chain-propagating step the chlorine atom abstracts one of the allylic hydrogen atoms. The radical that is produced in this step is called an allylic radical. - In the second chain-propagating step the allyl radical reacts with a molecule of chlorine - The chain reaction continues until the usual chain-terminating steps consume the radicals |
|
|
Allylic Bromination with N-Bromosuccinimide
|
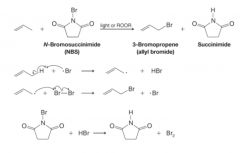
- Propene undergoes allylic bromination when it is treated with N-bromosuccinimide (NBS) in the presence of peroxides or light - The reaction is initiated by the formation of a small amount of Br radical - The main propagation steps for this reaction are the same as for allylic chlorination - N-Bromosuccinimide is a solid that provides a constant but very low concentration of bromine in the reaction mixture. |
|
|
Relative Stability of Radicals
|
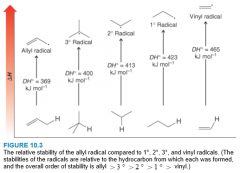
- The reason that allylic radicals are more stable than alkyl radicals is due to electron delocalization
|
|
|
Benzylic Group
|
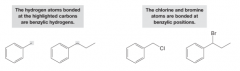
- An atom or group bonded to an sp3-hybridized carbon adjacent to a benzene ring - The group is said to be bonded at the benzylic position - Benzylic hydrogens are even more reactive than allylic hydrogens in radical substitution reactions due to the additional delocalization that is possible for a benzylic radical intermediate |
|
|
Anti-Markovnikov Addition of HBr
|

|
|
|
Radical Polymerization of Ethene
|
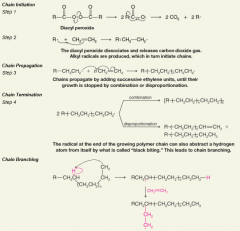
|
|
|
Ziegler-Natta catalysts
|
- organometallic complexes of transition metals - this process no radicals are produced, no back biting occurs, and, consequently, there is no chain branching - The polyethylene that is produced is of higher density, has a higher melting point, and has greater strength |
|
|
acid-catalyzed polymerizations
|
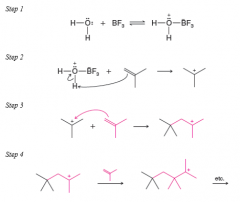
- growing chains in acid-catalyzed polymerizations are cations rather than radicals - The catalysts used for cationic polymerizations are usually Lewis acids that contain a small amount of water |
|
|
Polymerization of Alkenes with Electron-Withdrawing Groups
|
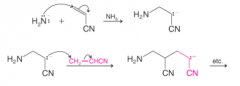
- polymerize in the presence of strong bases - Acrylonitrile, for example, polymerizes when it is treated with sodium amide (NaNH2) in liquid ammonia - The growing chains in this polymerization are anions |
|
|
Autooxidation
|
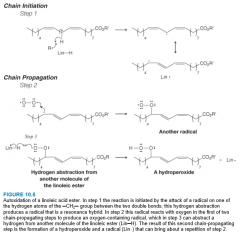
- The reaction of an organic compound with oxygen to form a hydroperoxide
|
|
|
Combustion of Alkanes
|
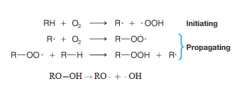
- When alkanes react with oxygen (e.g., in oil and gas furnaces and in internal combustion engines) a complex series of reactions takes place, ultimately converting the alkane to carbon dioxide and water. - the important reactions occur by radical chain mechanisms with chain-initiating and chain-propagating steps - One product of the second chain-propagating step is R─OOH, called an alkyl hydroperoxide. The oxygen-oxygen bond of an alkyl hydroperoxide is quite weak, and it can break and produce radicals that can initiate other chains: |

