![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
115 Cards in this Set
- Front
- Back
|
Charge of a proton |
+1.6×10^-19 C |
|
|
Charge of an electron |
-1.6×10^-19 C |
|
|
Mass of proton and neutrons |
1.67×10^-27 kg |
|
|
Mass of an electron kg |
9.11×10^-31 kg |
|
|
What is specific charge and unit |
The charge ÷ mass Unit= C kg-1 |
|
|
What must there be for a stable nucleus |
Strong nuclear forces to overcome electristatic forces of repulsion between protons in the nucleus to keep protons and neutrons together |
|
|
Does strong nuclear forces have the same effect on two protons as it does a proton and a neutron |
Yes |
|
|
What is the range of strong forces |
3-4 femtometres About diameter of small nucleus |
|
|
What is the range of electrostatic force of attraction between two charged particles? |
Infinite but decreases with range increase |
|
|
What is lowest range of strong forces |
0.5 fm |
|
|
What happens when strong forces are below 0.5 fm |
It's now a Repulsive force to prevent neurons and protons being pushed into eachover |
|
|
What is alpha decay |
●unstable nucleus releases an alpha particle |
|
|
What happens to atom after releasing alpha decay |
It's mass decreases by 4 and atom number reduces by 2 |
|
|
What is an alpha particle |
Two protons and 2 neutrons |
|
|
Alpha equation |
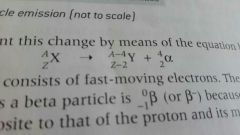
|
|
|
What is beta decay |
●consists of fast moving electrons ●unstable nucleus emits a beta particle |
|
|
What happens to an atom after releasing a beta particle |
● A neutron changes into a proton and releases beta particle ●mass stays the same but atom # +1 |
|
|
What is a beta particle |
●an electron ●mass of 0 ●charge of -1 (PUT WHERE ATOM # IS!!!) ●fast moving |
|
|
What is equation for beta decay |
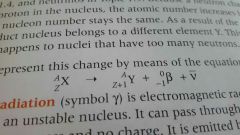
|
|
|
What else is released in beta decay |
●an anti particle with no charge ●called antineutrino |
|
|
What type of radiation is gamma radiation |
Electromagnetic radiation |
|
|
What can gamma radiation do ×3 |
●Pass through metal plates ●Has no mass or charge ●Elmsted by nucleus with too much energy following beta or alpha emissions |
|
|
What is the nucleon number |
●total of protons and neutrons ●atom mass |
|
|
What happens if there are less neutrons than stable isotope in an atom |
●it's less stable ●unstable nucleus may be radioactive and will decay over time into more stable nuclei |
|
|
How does radioactive isotopes get used on carbon dating |
●every organism has a certain amount of radioactive carbon - 14 ●this secretes and decays into more stable nuclei ●so scientists can judge how old it is by amount of C-14 |
|
|
What is specific charge of a particle |
It's ratio of charge to mass in coulomb per kilograms |
|
|
Equstion for specific charge |
Specific charge =charge ÷ mass |
|
|
Name two forces that are used to hold nucleon together |
●gravitational forces of attraction - very small ●electrostatic forces of repulsion |
|
|
Why are strong forces needed to hold together atoms |
●gravitational forces are not bog enough to counteract electrostatic forces of repulsion ●so another force is needed - the strong force |
|
|
4 things about the strong forces |
●they have a very short range of only a few fm ●there strength falls quickly beyond the short range ●they work equally between all nucleon attracting to eachover ie proton to protect etc ● they have small separations and are repulsive at some point or nucleon would crash into eachover |
|
|
What happens to strong forces bellow 0.5 fm |
They become repulsive to prevent nuleons collidig into eachover |
|
|
When does strong forces fall and become 0 |
After 3 fm |
|
|
Why does alpha emissions only occur in larger atoms |
●As big nucleus's are too massive for strong forces to keep them stable ●so they emit alpha particles to reduce size and become more stable |
|
|
What is range of alpha radiation |
●short ●only a few cm |
|
|
How do yoy measure alpha radiation |
●observing tracks in a cloud chamber ●moving a geiger counter away and towards |
|
|
What is beta emissions and in what type of atoms is it seen |
●Is the emission of an electron and an antineutrino ●seen only in neutron rich atoms as they are unstable due to proton neutron imbalance |
|
|
What happens to neutron when it emits beta radiation and what does it do to the particle that it came from |
●It splits onto an electron, an antineutrino and a proton ●thus increasing proton # by 1 and keeping nucleon number/ atom mass the same |
|
|
What is purpose of the small antineutrino |
Carries away some of the energy and momentum |
|
|
How where neutrinos found/ hypothesised |
●observed that not all energy was conserved during emission as electron did not have enough energy ●realised that another particle would be edited to take missing energy and momentum ●would have almost 0 mass and be neutral |
|
|
What is a photon and what does its size depend on |
●A photon is a packet of electromagnetic radiation ●photon size depends on frequency of radiation
|
|
|
What is there equaivalent to every particle (same mass, same rest every but opposite charge) |
An anti particle |
|
|
What is the rest energy of a particle |
● energy equivalent of a particles mass |
|
|
What is the equivalency of mass and energy and what equation is related to this |
●that energy can turn into mass and mass into energy ●remember to use mass energy ●e=mc^2 relates to this |
|
|
Explain equivalence of energy and mass in practise and what is it known as |
●if two protons collide you gwt a lot of energy at point of contact ● this can be converted into more particles ●if extra proton for example is produced so will an anti proton ●This is pair production |
|
|
What are antiparticles produced by (energy in the form of...) |
Photons |
|
|
How many photons needed to make a pair |
1 |
|
|
What wavelength photons only work in pair production |
Gamma |
|
|
What is the most common particle pair you gwt from a photon and why |
● electron and positron ●as they have a relatively low mass |
|
|
What is the minimum energy for a photon to undergo pair production |
●total rest energy of both particles produced Emin=hf min = 2E0 |
|
|
What is annihilation |
●when particle and antiparticles meet ●mass of both gets converted back into energy |
|
|
Why don't you get antiparticles in ordinary matter |
As they only exist for a fraction of a second before annihilation |
|
|
What causes a force |
●particle exchange ●when two particles interact something must happen to let one particle know the other one is there ●(It's caused by particle exchange) |
|
|
What are exchange particles |
●where particles cause a force ●think of a ball being thrown back and forth with people in water and think of a boomerang to attract |
|
|
What is repulsion between two protons caused by |
●exchange of virtual photons ● they are gauge bosons of the electromagnet force ●gauge bosons only exist for a short amount of time |
|
|
What are the four fundamental forces |
● strong nuclear forces ●weak nuclear forces ●electromagnetic force ●gravity |
|
|
What is the gauge bosons and what particles are effected by electromagnetic force |
●virtual photon (y) ●affects charged particles only |
|
|
What is boson and particles affected by weak forces |
●W+ W- ●affect all types of particles |
|
|
What is the gauge bosons and the particles affected by strong forces |
●pions pi+ pi- and pi0 ●hadrons only |
|
|
What does a larger mass do to gauge boson range |
●increasing the mass causes the range to be shorter ●due to fact more energy is needed to make it ●so only lasts a short time ●so can't travel far |
|
|
What is range of a photon of mass 0 |
Infinite |
|
|
What is a hadron |
●A particle that feels strong forces |
|
|
What are hadrons made from and what other from a of hadrons are there |
●made from quarks ●there are baryons and mesons |
|
|
Name two baryons and which one is most stable |
●neutron ●proton- most stable |
|
|
What does the fact that protons are the only stable baryons mean |
●that all others are unstable ●so decay to become other particles ●they must decay to become protons as they are the only stable baryons Explains beta - decay |
|
|
What is baryons number and is it all ways conserved |
●Number of baryons ●it's all ways conserved |
|
|
What interaction is beta decay caused by |
●weak interactions |
|
|
What is a pion ×2 |
●lightest meson ●three versions pi+ pi- and pi0 |
|
|
What is a kaon ×3 |
●heavier and more unstable than pion ●exist as K+ and K0 ●decay into pions die to short lifetime |
|
|
What force do baryons and mesons interact with eachover by |
●Strong force ●as they are both hadrons (only hadrons use string forces) |
|
|
What is a lepton and what type of interactions does it have |
●fundamental particles ●don't feel strong force as they aren't hadrons ●interact via weak interactions |
|
|
What is most stable lepton and why do mesons decay into them |
Electrons meaning that muons decay into them as they are not as stable |
|
|
What is mass and charge of the neutrinos muons and electrons have |
Zero electric charge and no mass |
|
|
What do yoy have to remember about lepton numbers |
●Le and L (weird u thing) are counted separately ●it's just number of leptons ●Each lepton has opposite antilepton |
|
|
What is strange quark and what property does it give to particles |
●let's you give strangeness property to particles |
|
|
When is strangeness conserved and not conserved |
●conserved when it's under strong interaction (when it's made under strong interactions) ●Not conserved when it decays under weak interactions |
|
|
What does the fact that strangeness mean to the quarks being produced in pairs and why |
●made in pairs to conserve strangeness ●eg kaons produce K+ (strangeness of +1) and K- (strangeness of -1) |
|
|
Charge of up down and strange quark and anti quarks |
●quarks= Up= +2/3 down=-1/3 strange=-1/3 ●Anti quarks= Ū=-2/3 đ=+1/3 ś=+1/3 |
|
|
Baryons number of quarks and anti quarks |
●quarks= same (+1/3) ●Anti quarks = same (-1/3) |
|
|
Strangeness of strange and anti strange quarks |
●strange = -1 ●Anti strange= +1 |
|
|
What are mesons made form |
●A quark and an anti quark ●eg kaons K + and K- |
|
|
What kind of interaction can change quark type? And give an example of it in beta + and - decay |
●weak interactions ●b+ proton changed by an unstable carbon 11 atom to form may neutron releasing a positron (anti electron and a neutrino) ●B- decay changes a day quark to a u releasing an electron and an antineutrino ~~~opposite to eachover~~~~~ |
|
|
Give 4 things that are conserved in particle interactions |
●charge ●momentum ●baryon number ●strangeness is conserved in strong interactions, but not weak |
|
|
Why can't quarks be free on their own |
● if you blast a proton with enough energy, the quarks energy just gets changes into more quarks and anti quarks ●pair production begins again ●just makes mesons |
|
|
What is it called when quarks that come from blasted protons firm mesons |
Quark confinement |
|
|
How are new theories validated |
●new theory is hypothesised ● describes new particle and properties u expect from it ●experiment then caried out to find the particle ●particle confirmed by many experiments done by different scientists ●theories more likely to be accepted so is validated |
|
|
Name an example of a new theory being validated |
●paul Dirac predicted antimatter ●this was proved by positron ●generally accepted ●however anti matter should be in equal amounts but it isn't meaning that there's something odd about it |
|
|
Issues with CERN |
●expensive ●scientists will need to collaborate ●lots of energy |
|
|
What is the photoelectric effect |
●beam of high frequency loght is shone at a metal sheet ●free electrons in the metal absorb photons ●if photon absorbed has enough energy to allow electron to break it's bond electron is released ●electrons broken off are photoelectron |
|
|
Give 3 conclusions of the photoelectric effect |
●no electrons are emitted if light is bellow a certain frequency called the threshold frequency ●photoelectrons are emitted with a range of kinetic energys from zero to max. Value of kinetic energy varies with frequency and is unaffected by light intensity ●Number of photoelectrons emitted is proportional to light intensity |
|
|
Can the photoelectric effect be explained by wave theory and why |
●Nope ●as it states that energy carried is proportional to intensity ●it says energy caried would be spread evenly over wavefront ●Each free electrons would gain a bit of energy ●gradually they would all have enough energy to leave the metal |
|
|
What did explain photoelectric effect and why |
●Einstein photon model of light ●as it says that when light hiya surface metal is bombarded by photons ●one of these photos collides with an electron and electron will gain it's energy |
|
|
Equation that relates to Einstein photon model of light |
E=hf=hc÷lander |
|
|
What is energy required to break the electrons bond in photoelectric effect |
Work function |
|
|
How does the photon model explain threshold frequency |
●if energy gained from the photon is greater than the work function electron is emitted ●if it isn't no electron will be emitted |
|
|
Equation for threshold frequency |
f=work function ÷ planks constant |
|
|
How does photon model of wave explain max kinetic energy |
●energy transfered to an electron is hf ●kinetic energy is hf minus any lost ●minimum amount of energy that can be lost is the work function and the max is given by hf= work function + Ek max |
|
|
Why so there a range of energies in the metal |
●As the deeper electrons loose more energy than those at the surface |
|
|
What does stopping potential help us measure in the photoelectric effect and it's equation |
●maximum kinetic energy ●as stopping potential is the p.d needed to stop the fastest moving electrons. ●so work done to stop the electron us the max kinetic energy ●given by eV=Ek max |
|
|
What happens when electrons move down an ergonomic level on the atom |
●It releases a photon that must correspond with the sane energy inbetween the energy levels (specific amount) |
|
|
What happens during excitation |
●when an electron absorbes a photon and moves up an energy level |
|
|
What must energy of photon emmited by an atom in moving electron down an energy level be equal to |
Difference in energies between the two levels |
|
|
What is the ionisation energy |
Energy needed to remove an electron form it's ground state n=1 |
|
|
How does a fluorescent tube cause light |
●contain Mercury gas and a high voltage is put across it causing fast moving free electrons that ionise some of the Mercury atoms ●this produces more free electrons that collide with other Mercury atoms causing the Mercury atoms to become excited to higher energy levels ●when these reach their ground states they emmited uv photons ●uv photons are absorbed by phosphorus coating causing it's elections to get excited to higher energy levels ●these then cascade down to lower energy levels releasing lower energy photons in the form of visual effects light |
|
|
What does each line in fluorescent tube emission spectra represent and what does it mean about energy |
●A specific wavelength ●wavelength corresponds to the photon energies as only certain photon energies are allowed |
|
|
What is a continous spectra of light and why is it caused |
●where you split up light from a prism and there is no gaps in the spectrum ● as all the wavelengths are allowed as the electrons are not confined to energy levels in the object producing the continous spectrum |
|
|
What do you see on light absorption spectra whith visible light |

|
|
|
What does a cool gas do to certain wave lengths when light is passed through it and into a prism |
● removes certain wavelengths ●as most electrons will be in ground states ●so photons will be absorbed by electrons (only those equal to the gap between energy levels) ●so those absorbed wavelengths will be missing from the spectra ●black lines = absorbed wavelengths |
|
|
What is an emission spectra ( and compare to absorption spectra) |
● where you have excited specific gas . ●black lines of absorption lone up with bright form emission spectra |
|
|
Whare did de broglie say about wave particle duality |
●that wave like light shows particle like properties and that particles such as electrons show wave like properties |
|
|
De broglie equation |
Lander=h÷mv |
|
|
What shows wave nature of electrons |
●electron diffraction ●accelerated electrons are fired in vacuum tube and interact with spaces in graphite crystal structure ●shows electrons have wave like properties as slower electrons give wider spaced rings and increasing electron speed squashes the pattern toward the middle |
|
|
What is size of wave for electrons in a vacuum tube equal to in the wave spectrum |
X ray |
|
|
What happens when there is a larger mass and momentum at the same speed in the electron diffraction demonstration |
●they have a more tightly packed diffraction pattern ●for example neutrons have mass greater than electrons so have a shorter de broglie wavelength |
|
|
When doesn't a particle show wave like properties |
●won't get diffraction when object that particle interacts with is bigger than its de broglie wavelength |
|
|
How was wave particle duality accepted |
● when it was validated ●(wasn't first excepted by other scientists) ●will be excreted until new evidence comes along and conflicts it |

