![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
38 Cards in this Set
- Front
- Back
|
wavelength (λ)
|
The distance between identical points on successive waves
|
|
|
frequency (ν)
|
The number of times per second that one complete wavelength passes a given point
|
|
|
Planck’s constant (h)
|
The constant that relates the energy and frequency of a photon, E = hν. Its value is 6.662 X 10⁻³⁴J/s
|
|
|
photoelectric effect
|
The emission of electrons from a metal surface induced by light
|
|
|
photons
|
The smallest increment (a quantum) of radiant energy; a photon of light with frequency v has an enegy equal to hv
|
|
|
spectrum
|
The distribution among various wavelengths of the radiant energy emitted or absorbed by an object
|
|
|
continuous spectrum
|
A spectrum that contains radiation distributed over all wavelengths
|
|
|
line spectrum
|
A spectrum that contains radiation at only certain specific wavelengths
|
|
|
ground state
|
The lowest-energy, or most stable, state
|
|
|
excited state
|
A higher energy state than the ground state.
|
|
|
matter waves
|
The term used to describe the wave characteristics of a moving particle
|
|
|
uncertainty principle
|
A principle stating there is an inherent uncertainty in the precision with which we can simultaneously specify the position and momentum of a particle. This uncertainty is significant only for particles of extremely small mass, such as electrons
|
|
|
wave function
|
A mathematical description of an allowed energy state (an orbital) for an electron in the quantum mechanical model of the atom; it is usually symbolized by the Greek letter ψ
|
|
|
probability density
|
(ψ²) A value that represents the probability that an electron will be found at a given point in space. Also called electron density
|
|
|
electron density
|
The probability of finding an electron at any particular point in an atom; this probability is equal to ψ², the square of the wave function. Also called the probability density
|
|
|
orbital
|
An allowed energy state of an electron in
the quantum mechanical model of the atom; the term orbital is also used to describe the spatial distribution of the electron.An orbital is defined by the values of three quantum numbers: n, l, and mₗ |
|
|
electron shell
|
A collection of orbitals that have the same value of n. For example, the orbitals with n = 3 (the 3s, 3p, and 3d orbitals) comprise the third shell.
|
|
|
subshell
|
One or more orbitals with the same set of quantum numbers n and l. For example,we speak of the 2p subshell (n = 2, l = 1), which is composed of three orbitals (2pₓ, 2p(subscript)y, 2p(subscript)z)
|
|
|
radial probability function
|
The probability that the electron will be found at a certain distance from the nucleus
|
|
|
node
|
Points in an atom at which the electron density is zero. For example, the node in a 2s orbital is a spherical surface
|
|
|
degenerate
|
A situation in which two or more orbitals have the same energy
|
|
|
spin magnetic quantum number (mₛ)
|
A quantum number associated with the electron spin; it may have values of +1/2 or -1/2
|
|
|
Pauli exclusion principle
|
A rule stating that no two electrons in an atom may have the same four quantum numbers (n, l, mₗ, and mₛ, and ms ). As a reflection of this principle, there can be no more than two electrons in any one atomic orbital.
|
|
|
electron configuration
|
The arrangement of electrons in the orbitals of an atom or molecule
|
|
|
Hund’s rule
|
A rule stating that electrons occupy degenerate orbitals in such a way as to maximize the number of electrons with the same spin. In other words, each orbital has one electron placed in it before pairing of electrons in orbitals occurs.
|
|
|
valence electrons
|
The outermost electrons of an atom; those that occupy orbitals not occupied in the nearest noble-gas element of lower atomic number. The valence electrons are the ones the atom uses in bonding
|
|
|
core electrons
|
The electrons that are not in the outermost shell of an atom
|
|
|
representative (main-group) element
|
An element from within the s and p blocks of the periodic table
|
|
|
transition elements
|
(transition metals)Elements in which the d orbitals are partially occupied.
|
|
|
lanthanide (rare earth) element
|
Element in which the 4f subshell is only partially occupied.
|
|
|
f-block metals
|
Lanthanide and actinide elements in which the 4f or 5f orbitals are partially occupied.
|
|
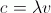
|
light as a wave: c = speed of light (3.00 X 10⁸ m/s) λ = wavelength in meters, v = frequency in s⁻¹
|
|
![<div><div><div>[latex]\[\small E = hv\][/latex]</div></div></div><div><br /></div>](https://images.cram.com/images/upload-flashcards/1086145/2318989_m.gif)
|
light as a particle (photon): E = energy of photon in joules, h = Planck’s constant (6.626 X 10⁻³⁴ J-s) n = frequency in s⁻¹
|
|
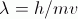
|
matter as a wave: λ = wavelength in meters, h = Planck’s constant m = mass of object in kg, v = speed of object in m/s
|
|
![[latex]<div>\[\small \Delta x \cdot \Delta (mv) \ge = \frac{h}{4 \pi}\]</div><div></div>[/latex]](https://images.cram.com/images/upload-flashcards/1086145/2318557_m.gif)
|
Heisenberg’s uncertainty principle. The uncertainty in position Δx and momemtum Δmv of an object cannot be zero; the smallest value of their product is h/ 4π
|
|
|
electronic structure
|
The arrangement of electrons in an atom or molecule
|
|
|
electromagnetic radiation
|
(radiant energy)
A form of energy that has wave characteristics and that propagates through a vacuum at the characteristic speed of 3.00 X 10⁸ m/s |
|
|
∆E = −R(subscript H) ( 1/n²[subscript f] -1/n²[subscript i] )
|
The energy absorbed or emitted from the process of electron promotion or demotion;
where R(sub H) is the Rydberg constant, 2.18 × 10 ⁻¹⁸ J, and ni and nf are the initial and final energy levels of the electron |

