![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
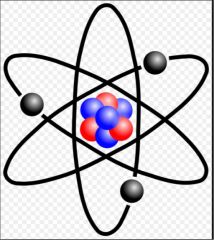
Atom |
The basic unit of matter (34) |
|
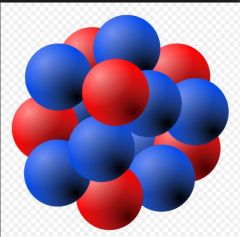
Nucleus |
The center of an atom, which contains the protons and neutrons. (34) |
|
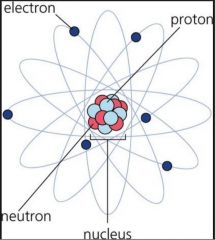
Electron |
Negatively charged particles; located in the space surrounding the nucleus (34) |
|

Element |
Pure substance that consists entirely of one type of atom (35) |
|
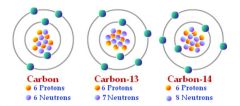
Isotope |
One of several forms of a single element, which contains the same number of protons but different numbers of neutrons (35) |
|
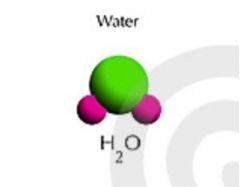
Compound |
Substance formed by the chemical combination of two or more elements in definite proportions (36) |
|
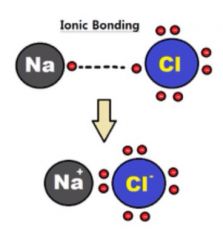
Ionic Bond |
Chemical bond formed when one or more electrons are transferred from one atom to another (37) |
|
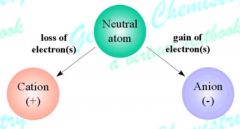
Ion |
Atom that has a positive or negative charge (37) |
|
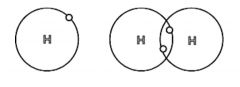
Covalent Bond |
Type of bond between atoms in which the electrons are shared (37) |
|
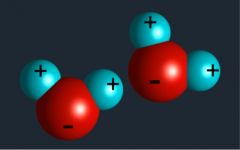
Molecule |
Smallest unit of most compounds that displays all the properties of that compound (37) |
|

Van Der Waals Forces |
Slight attraction that develops between oppositely charged regions of nearby molecules (38) |

