![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
53 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
To carry out its functions a cell needs |
Constan supply of reactants (nutrients and oxygen) also needs system to remove waste products (carbon dioxide and water) |
|
|
|
Diffusion |
Reactants can diffuse through cell membrane. Waste products can diffuse out and be absorbed by surrounding liquid. |
|
|
|
Body composed of |
42 L of fluids on average (2/3 of body weight) |
|
|
|
Two categories of body fluids |
Intracellular and extracellular fluid. |
|
|
|
Intracellular fluid |
Located inside cells. Majority of fluids are here *28L |
|
|
|
Extracellular fluids |
Located outside cells. Transports substances to and from cells |
|
|
|
Two categories of extracellular fluids |
1. Interstitial fluid surround individual cells and moves in lymph vessels (10.5L to 20% of total fluid) 2. Plasma- the liquid portion of blood (3.5L- 7% of total) •other body fluids are in lesser amounts and are urine, juices, cerebrospinal fluid |
|
|
|
Body fluids composition |
Intracellular fluids contain more protein than extracellular fluids and different ionic populations. |
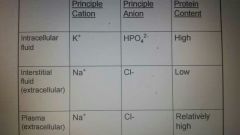
|
|
|
98% of the oxygen needed |
By the body is carried by red blood cells. Other 2% dissolved in plasma due to low solubility of O2 in plasma. |
|
|
|
Oxygen reversibly binds |
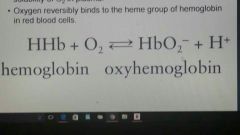
To the heme group of hemoglobin in red blood cells |
|
|
|
Hemoglobin has |
4 chains and 4 hemes. 4 O2 can bind. Cooperative protein - binding of one O2 increases affinity for other O2 |
|
|
|
Heme structure |
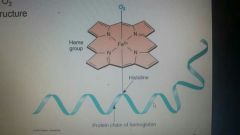
|
|
|
|
When CO2 is present |
Hemoglobin can reversibly bind it. CO2 is transported in blood. 25% carried out by carbaminohemoglobin from body tissues to lungs. 5% dissolved in plasma 70% as HCO3- ions |
|
|
|
Transport of oxygen and CO2 to and from the Lungs |

|
|
|
|
CO2 and O2 |
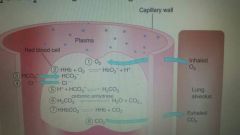
Exchange at the lungs |
|
|
|
Transport of oxygen and CO2 to and from the cells of active tissue |

|

|
|
|
CO2 and O2 exchange |
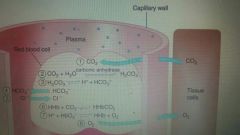
At cell |
|
|
|
Chemical transport to cells |
To be transported in the bloodstream, chemicals can 1. Dissolve in plasma (sugars and ions) 2. Bind to cellular components (O2 and CO2) 3. Form a suspension (lipids) |
|
|
|
Chemical transport across capillaries |

|

|
|
|
Constituents of urine |
Urine is about 96% water and 4%dissolved organic and inorganic waste products. • can be used diagnostically( ph,alkalinity, or acidity) • Normal constituents are considered abnormal when they are eliminated in excess. • |
|
|
|
Glucosuria |
Diabetes mellitus, renal diabetes. Alimentary glycosurina. Glucose in large amounts |
|
|
|
Proteinuria (albuminuria) |
Kidney damage, nephritis, bladder infection. Protein. |
|
|
|
Ketonuria |
Diabetes mellitus, starvation, high fat diets. Ketone bodies |
|
|
|
Pus |
Kidney or bladder infection |
|
|
|
Hemoglobinuria |
Excessive hemolysis of red blood celss. Hemoglobin |
|
|
|
Hematuria |
Hemorrhage in the urinary tract. Ted blood cells |
|
|
|
Jaundice |
Blockage of bile duct, hepatitis, cirrhosis. Bile pigments in large amounts |
|
|
|
Fluid and electrolyte balance |
Interdpendent, If one deviates from normal so does the other. Hospital patients given electrolyte and fluid balance therapy. Water input must equal water loss |
|
|
|
Water intake regulated by |
Thirst mechanism. •salivary secretions decrease when body loses water. • makes you thirsty and you drink water. |
|
|
|
Fluid balance in body maintained |
Or restored primarily by variation in urine output |
|
|
|
The rate of water reabsorption |
From the renal tubules in the kidneys can be varied to adjust for water intake. |
|
|
|
Renal tubles |
Small structures in the kidney that filter the blood and produce the urine |
|
|
|
Water reabsorption is controlled by |
The pituitary hormone vasopressin And the adrenal cortex hormone aldosterone |
|
|
|
Vasopressin |
Increases reabsorption of water from renal tubules and into the capillaries increasing blood vol. |
|
|
|
When bodily fluid levels |
Run really low due to not drinking enough water, diarrhea or excessive perspiration. Aldoesterone is secreted which then stimulates the readsorption of Na+ at the kidney from the renal tubules into the blood. •Chloride ions follow the sodium ions to maintain electric neutrality and water follows the NaCl • therefore, both water and salt concentration are conserved |
|
|
|
Normal blood ph |
Range of 7.35-7.45 |
|
|
|
Alkalosis |
Higher than normal blood pH |
|
|
|
Acidosis |
Lower than normal blood pH |
|
|
|
Three systems to control blood pH |
Buffer, respiratory, urinary |
|
|
|
Buffer control of blood pH |
3 major buffer systems of the blood. 1. Bicarbonate buffer 2. Phosphate buffer 3. Plasma proteins Buffers work by neutralizing H+ and Oh ions that come from dissociation of acids or bases. |
|
|
|
Respiratory control of blood pH |
Respiration helps control blood pH by regulating the elimination of CO2 and H2O. Come from break down of carbonic acid • the more CO2 and H2O exhaled, the more carbonic acid is removed. Raising blood pH. |

|
|
|
Respiratory control of blood pH cont |
Begins in the brain with respiratory center neurons that are sentitive to co2 and pH |
|
|
|
Hyperventilation |
Rapid deep berthing. Causes an increase in blood pH when pH level is lower than normal or high CO2 •increased breathing eliminates more CO2, reduces amount of carbonic acid and H+ and increases pH to normal. |
|
|
|
Hypoventilation |
Slow, shallow breathing causes a decrease in blood pH when pH level is above normal. •less CO2 exhaled, higher concentration of carbonic acid remains and lowers pH to normal. |
|
|
|
Urinary control of blood pH |
Kidneys can excrete varying amounts of acidic and base so they can control pH • the kidneys when the blood is acidic, can excrete H+ ions in the urine decreasing it's pH and raising blood pH |
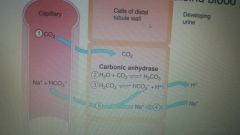
|
|
|
Reactions involved in the excretion of hydrogen ions |

|
|
|
|
Maintain ratio of biocarbonate |
To carbonic acid in plasma (20-1 normal condition) |
|
|
|
Respiratory and urinary systems |
Work together to maintain pH by maintaining this ratio. Change ratio change pH. |
|
|
|
pH imbalances |
In body fluids are brought on by: 1. abnormal breathing (acidosis or alkalosis) 2.other factors (metabolic acidosis or alkalosis) Increase ratio=alkalosis Decrease ration= acidosis |
|
|
|
Respiratory alkalosis |

Hyperventilation increases CO2 loss, this increasing blood pH. Loss of CO2 |
|
|
|
Respiratory acidosis |

Hypoventilation (slow shallow breathing.) Decreases CO2 loss, thus decreasing blood pH |
|
|
|
Metabolic acidosis |

An increase in h+ in bodily fluids caused by diabetes mellitus, heavy exercise, acids developed by metabolic processes. Leads to lower blood pH. When H+ diffuses into blood. |
|
|
|
Metabolic alkalosis |
A decrease in H+ in bodily fluids caused by: Vomiting (loss of stomach acid) :ingestion of alkaline substances. Leads to an increase in blood pH •body responds by slowing breathing (hypoventilation) |
|

