![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Spontaneous Process |

A process that does occur under a specific set of conditions. |
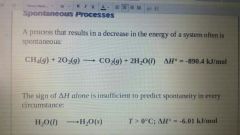
A reaction that results in a Decrease in the energy of a system often is spontaneous. |
|
|
Nonspontaneous |
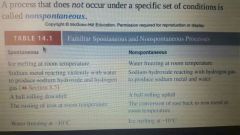
A process that does not occur under a specific set of conditions |
|
|
|
Delta S system. |

The difference in entropy of the final State and the entropy of the initial state. |
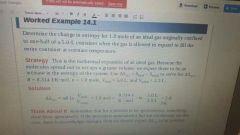
|
|
|
Standard Entropy |
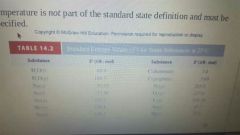
The absolute entropy of a substance at 1 atm. Temp must be specified. |
|
|
|
Trends in Entropy |

|
|
|
|
Delta S rxn |

|

|
|
|
Increase in Entropy |
Several Processes that lead to an increase in entropy are. Melting, Vaporization or Sublimation, Temperature increase, Reaction resulting in a greater number of gas molecules. |

|
|
|
Second Law of Thermodynamics |

States that for a process to be spontaneous Delta S universe must be positive. |

|
|
|
Example: Second Law of Thermodynamics |

|

|
|
|
Third Law of Thermodynamics |
States that the entropy of a perfect crystalline substance is zero at absolute serotonin. |

Entropy Increases in a substance as temperature increases from absolute zero. |
|
|
Gibbs Free Energy |

|

|

