![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
List the following levels of the biological hierarchy in order of increasing magnitude: molecules, particles, cells, atoms, organelles.
|
Particles < Atoms < Molecules < Organelles < Cells
|
|
|
What are the four elements that make up 96% of a living organism?
|
Oxygen (65%)
Carbon (18.5%) Hydrogen (9.5%) Nitrogen (3.3%) |
|
|
What are four other relevant elements for this class?
|
Phosphorus
Sulfur Sodium Chlorine |
|
|
Composition of an Atom
|
Three types of particles:
-protons (positive, heavy, nucleus) -neutrons (neutral, heavy, nucleus) -electrons (negative, light, orbitals) Overall positive charge in nucleus. Electrons (-) are attracted to nucleus. |
|
|
Shell Model
|
-Electrons fill the innermost shell first.
-innermost shell can hold up to 2 electrons -next shell can hold up to 8 electrons -valence shell: whatever shell is the outermost shell (contains valence electrons) -It takes E to move an electron from an inner shell to an outer shell. -It releases E to move an electron from an outer shell to an inner shell. |
|
|
Atomic Number vs. Atomic Mass/Mass Number/Atomic Weight
|
Atomic Number: number of protons in the nucleus
Atomic Mass/Mass Number/Atomic Weight: sum of protons and neutrons |
|
|
Ions
|
-charged atoms
-result of gain/loss of an electron -cause change in charge but not in atomic mass -cation: overall pos. charge -anion: overal neg. charge |
|
|
Isotopes
|
-atoms with different numbers of neutrons
-result of extra neutron(s) being added to nucleus -unstable, energy is released (radioactivity) -affects atomic mass but not charge |
|
|
Molecules
|
-2 or more atoms joined together
- two general ways that atoms interact = sharing of electrons and charge-based bonds |
|
|
Covalent Bonds
|
-occur because of sharing of electrons between atoms
-When sharing of electrons between atoms is not equal (i.e. between atoms which have large differences in EN; atom with higher electronegativity more strongly attracts electrons), POLAR COVALENT BONDS form. -When sharing of electrons is equal (i.e. EN difference is not that large), NONPOLAR COVALENT BONDS form. |
|
|
Types of Charge-Based Bonds
|
Ionic Bonds
Hydrogen Bonds van der Waals interactions |
|
|
Ionic Bonds
|
-strongest type of charge-based bond
-occur as a result of interaction between ions -e.g. NaCl |
|
|
Hydrogen Bonds
|
-weaker than ionic bonds
-occur as a result of H atoms covalently bonding to highly EN elements (such as O) -the resulting weak charge allows charge-based bonding b/t atoms/molecules -inTERmolecular -e.g. water |
|
|
van der Waals Interactions
|
-weakest charge-based bond
-occur when electrons randomly cluster in regions of atom, resulting in local areas of slight neg. and slight pos. charge |
|
|
Properties of Water
|
-dual charge makes water an important molecule
-interacts well with any charged atom/molecule -is a great solvent (anything with a charge, i.e. polar, will dissolve in it) -cohesion: attraction of water to other water molecules -adhesion: attraction of water to other molecules/surfaces -H and O are held together via a polar covalent bond, because O has a higher EN than H, and therefore it attracts Hydrogen's electrons. This results in a partial positive charge around the Hydrogens and a partial negative charge around the Oxygen. -Water molecules interact via Hydrogen bonding. |
|
|
Acid/Base Scale
|
-Pure water is neutral. [H+] = 10^-7 mol/L
-If [H+] > 10^-7 mol/L ACIDIC (0-6.9) -If [H+] < 10^-7 mol/L BASIC (7.1-14) -If [H+] = 10^-7 mol/L NEUTRAL (7) -pH scale ranges from 0-14 -pH = -log[H+] |
|
|
Acid vs. Base
|
Acid
-pH 0-6.9 -increases [H+] in a soln when added Base -pH 7.1-14 -decreases [H+] in a soln when added |
|
|
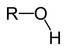
Hydroxyl Functional Group
Charged due to polar covalent bond Can act as a weak acid |
|
|
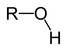
Hydroxyl Functional Group
Charged due to polar covalent bond Can act as a weak acid |
|
|
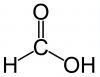
Carboxyl Functional Group
Acts as an acid |
|
|
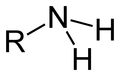
Amine Functional Group
Acts as a base |
|
|
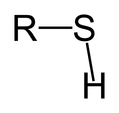
Sulfhydryl Functional Group
Can form disulfide bonds with another SH |
|
|
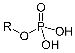
Phosphate Functional Group
Tends to be negative to to loss of H+ |
|
|
Macromolecules
|
-large molecules, bonds hold them together
-join monomers together to make polymers -covalent linking by dehydration/condensation reaction -breaking of macromolecule core bonds often involves hydrolysis reactions |
|
|
Four Classes of Macromolecules
|
Carbohydrates (sugars, starch)
Lipids (fats, oils) Proteins Nucleic Acids (DNA, RNA) |
|
|
Condensation Reaction
|
A-OH HO-B
(H and O bond to form water) A-O-B + H2O |
|
|
Hydrolysis
|
A-O-B + H2O
(water breaks bond) A-OH HO-B |
|
|
Carbohydrates
|
-energy source, cell identification, structurally important
-contain C, H, O -simple forms usually in ratio: (CH2O)n (i.e. 1:2:1) -monomers: monosaccharides -vary by # of carbons (e.g. triose=C3H6O2, pentose=C5H10O5, hexose=C6H12O6) -polymers: polysaccharides (2+ monosaccharides joined together; glycosidic bonds) -names based on # of monomers joined together (e.g. disaccharides=2 monosaccharides, trisaccharides=3 monosaccharides) -some important polysaccharides: starch (long glucose chains in plants, energy storage), glycogen (long glucose chains in animals, energy storage), cellulose (long glucose chains in plants, structural) |
|
|
Lipids
|
-long CH chains/rings (covalently bonded)
-very hydrophobic due to nonpolarity -how hydrophobic the molecule is is dependent on the length of the chain/size of ring -functions: cell signaling, energy storage, membrane components -components: fatty acids, triglycerides, phospholipids, steroids |
|
|
Fatty Acids
|
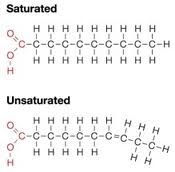
-CH-rich chains with a -COOH at the end
-chains of FA can be saturated or unsaturated |
|
|
Triglycerides
|
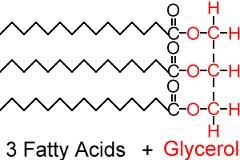
-3 FA chains linked onto glycerol
-type of lipid |
|
|
Phospholipids
|
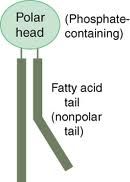
-2 FA chains + 1 Phosphate (-) group attached to glycerol backbone
-type of lipid -amphipathic: hydrophobic on one side of molecule, hydrophilic on other side of molecule |
|
|
Steroids
|
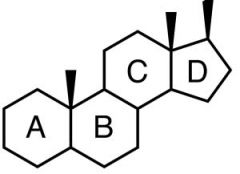
-very hydrophobic
-ringed -CH-rich -nonpolar covalent bonds -functions: signals in cell (and b/t cells), cross membranes easily |
|
|
Saturated vs. Unsaturated
|
Saturated: each C is bonded to the maximum # of Hs possible; no double bonds
Unsaturated: asymmetrical; double/triple bonds can exist |
|
|
Proteins
|
-structural ("-in"), enzymatic ("-ase")
-monomer: amino acids (AAs) -R groups determine the unique properties of different AAs -If hydrophobic, R groups are CH-rich, nonpolar covalent bonds. -If hydrophilic, R groups polar, ionic bonds. -polymer: polypeptide (i.e. a protein) = 2+ covalently bonded AAs (hydrolysis occurs between two AAs, allowing AAs to bond to each other and form a polypeptide) -peptide grows from N terminus --> C terminus -N-terminus of new AA is added to (i.e. bonded) to C-terminus of growing polypeptide -have four levels of structure; many are active at tertiary level, but not all |
|
|
Amino Acid
|
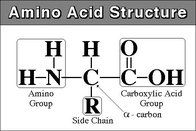
-monomer of protein
-end with amine functional group is "N-terminus" -end with carboxyl functional group is "C-terminus" |
|
|
Levels of Structure for Proteins
|
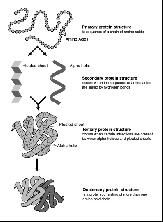
*Primary:
-the chain of AAs in linear form; describes ordering of specific AAs **Secondary: -primary structure folds on itself due to H-bonding -two main forms -- alpha helix and beta sheet ***Tertiary Structure: -more folding of secondary structures to make a 3D structure -ionic bonding, H-bonding, covalent bonding, van der Waals interactions -many proteins are active at this level, but not all ****Quaternary Structure: -2+ individual tertiary-level polypeptides that interact via all bond types |
|
|
Denaturation vs. Renaturation
|
-Denaturation: unfolding of protein (b/c of heat breaking bonds)
-Renaturation: re-folding of denatured proteins |
|
|
Active Proteins
|
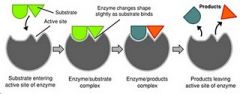
-active once they have reached tertiary or quaternary structure
-can be structural ("-in") or enzymatic ("-ase") -enzyme + substrate(s) -Specific substrates fit into active sites of specific enzymes. The enzyme covalently bonds these substrates and releases them (or process can go in reverse direction). |
|
|
Nucleic Acids
|
-hold genetic information
-monomers: nucleotides -nucleotides contain a pentose sugar, a negatively charged phosphate group, and a nitrogenous base -polymer: DNA, RNA -To make the polymer, nucleotides get added to 3' end of growing chain and covalent bonds form between 5' PO4(-) and 3'OH on adjacent nucleotides. -nucleoside: sugar attached to base (no phosphate group) |
|
|
DNA Properties
|
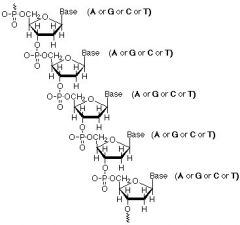
Sugar: DEOXYribose (H on 2' Carbon, NOT OH)
Phosphate (on 5' Carbon): PO4(-) Nitrogenous Bases (on 1' Carbon): Adenine (purine, two-ring), Guanine (purine, two-ring), Cytosine (pyramidine, one-ring), Thymine (pyramidine, one-ring) |
|
|
RNA Properties
|
sugar: ribose (OH on 2'C, NOT H on 2'C)
Phosphate (on 5' end): PO4(-) Nitrogenous Bases (on 1'C): Adenine (purine, two-ring), Guanine (purine, two-ring), Cytosine (pyrimidine, one-ring), Uracil (pyrimidine, one-ring) |
|
|
Key macromolecule of cell membrane
|
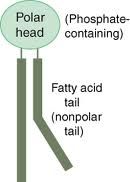
-Phospholipid
-phosphate group (neg. charge) attached to glycerol attached to two fatty acids (no charge) |
|
|
If you toss phospholipids into water, two things can happen...
|
-form a micelle
-form a bilayer |
|
|
Micelle
|
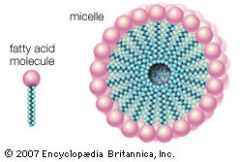
-one possibility when phospholipids encounter water
-polar/hydrophilic heads on outside, nonpolar/hydrophobic tails on inside |
|
|
Bilayer/Liposome
|
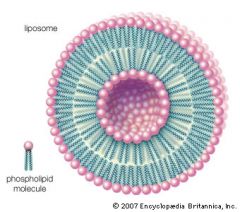
-one possibility when phospholipids are thrown into water
-polar/hydrophilic heads on outside and inside of ring, nonpolar/hydrophobic tails meet between two layers of polar heads |
|
|
Phospholipid Bilayer Structure
|
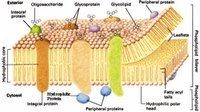
|
|
|
Phospholipid Bilayer Details
|
*Components:
-Phospholipids (2 layers) -Proteins -Integral: have a strongly hydrophobic region that makes protein sit IN membrane -Peripheral: primarily hydrophilic -Carbohydrates (used as cell markers) -Glycoproteins: attached to proteins -Glycolipids: attached to lipids -Other Lipids -Cholesterol: ringed, HC-rich, nonpolar; breaks up stacking of FAs, keeping membrane fluid |
|
|
What crosses the membrane easily vs. what doesn't
|
DOES: gases, smaller uncharged molecules, water, steroids
DOESN'T: ions, big bulky molecules (sugars) |
|
|
Diffusion
|
-molecules naturally want to flow from areas of high concentration to areas of low concentration
-requires no energy input to make this happen -gradient: adjacent regions of high concentration and low concentration |
|
|
Facilitated Diffusion
|
-Diffusion with the aid of a protein
-Proteins facilitate movement of ions (etc.) across the membrane. |
|
|
Osmosis
|
-water movement across membrane
|
|
|
Hypertonic Solution
|
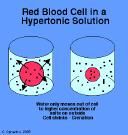
-concentration outside of cell is greater than inside cell
-water flows out, cell shrinks |
|
|
Isotonic Solution
|
-concentrations are equal inside and out
-no net water flow |
|
|
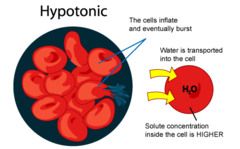
Hypotonic Solution
|
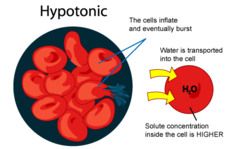
-concentration outside cell is less than concentration inside cell
-water flows in, cells swell |

