![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
1466 Cards in this Set
- Front
- Back
|
Interaction between the nervous system and the endocrine system is coordinated through the
|
hypothalamus
|
|
|
Signaling mechanism where a neuron releases a chemical mediator into one of both of the vascular systems, which will affect a cell at some distance
|
neuroendocrine
|
|
|
Characteristics of endocrine glands
|
ductless, very vascular (fenestrated and sinusoidal capillaries).
|
|
|
Primary stromal fibers of endocrine glands are
|
reticular fibers
|
|
|
Fibers of endocrine gland capsule
|
Dense, irregular type I collagen
|
|
|
Endocrine gland parenchyma arrangement (4)
|
1. Unicellular (diffuse neuroendocrine system)
2. Cords - cells line up in rows 3. Follicular pattern 4. Multipolar neurons |
|
|
Lumen of endocrine gland stores how many days worth of hormone precursors?
|
30
|
|
|
Steroid secreting glands store
|
cholesterol (hormone precursor)
|
|
|
Peptide hormones are 1) first produced as ... 2) then converted in the ER to ... 3) then packaged into secretory vesicles in the Golgi where it becomes
|
1) preprohormone
2) prohormone 3) active hormone |
|
|
Where is angiotensin I (inactive) is converted to angiotensin II (active)?
|
In the blood
|
|
|
A change in the number or affinity of receptors is called
|
down- or up-regulation
|
|
|
Down-regulation may occur by (3)
|
1) decreasing the synthesis of new receptors
2) increasing the degradation of existing receptors 3) inactivating receptors |
|
|
Up-regulation may occur by (3)
|
1) increasing synthesis of new receptors
2) decreasing degradation of existing receptors 3) activating receptors |
|
|
If a response reverses the original stimulus, this is called
|
a negative feedback system (loop)
|
|
|
Three types of closed negative feedback loops
|
1) Ultrashort (autocrine) - Cell A feeds back to Cell A
2) Short (most common) - Cell A produces a mediator which affects Cell B, which produces a feedback to Cell A 3) Long - Cell B produces a mediator which affects Cell C, which in turn feeds back to both cell A and cell B |
|
|
Tyrosine derivatives are
|
epinephrine & norepinephrine - adrenal medulla
T3 & T4 - Thyroid gland |
|
|
Derivatives of tryptophan
|
melatonin (pineal gland), and serotonin
|
|
|
Small peptides
|
ADH and oxytocin (posterior pituitary)
ANP (heart atria) |
|
|
Small proteins
|
glucagon, insulin, somatostatin, pancreatic polypeptide - pancreas
All anterior pituitary hormones - anterior pituitary PTH - parathyroid glands |
|
|
Steriods
|
aldosterone and cortisol - adrenal cortex
estrogen and progesterone - ovaries testosterone - testes |
|
|
Eicosanoids
|
prostaglandins and leukotrienes - all cells except red blood cells
|
|
|
Gas
|
nitric oxide - endothelial cells lining blood vessels
|
|
|
Hormones become permanently inactive ...
|
in their target tissue
|
|
|
The actions of some hormones on target cells require a simultaneous or recent exposure to a second hormone
|
permissive effect
|
|
|
When the effect of two hormones acting together is greater or more extensive than the effect of each hormone acting alone, the two hormones are said to have a
|
synergistic effect
|
|
|
When one hormone opposes the actions of another hormone, the two hormones are said to have
|
antagonistic effects
|
|
|
Endocrine disorders can arise from (2)
|
1) hyperfunction or hypofunction of endocrine organs (most disorders)
2) excessive or inadequate responsiveness to target cells to hormones that are produced |
|
|
The hypophysis (pituitary gland) has two major divisions
|
1) anterior adenohypophysis
2) posterior neurohypophysis |
|
|
Neurohypophysis is derived from
|
neuroectoderm
|
|
|
Neurohypophysis have 2 parts
|
1) Pars nervosa (posterior, or neural, lobe)
2) Infundibulum = Infundibular stem and median eminence |
|
|
Adenohyphosis is derived from
|
epithelial tissue
|
|
|
Adenohypophysis is made up of three parts
|
1) Pars distalis (anterior lobe)
2) Pars intermedia - absent in adults 3) Pars tuberalis |
|
|
The anterior and posterior pituitary lies in a bony fossa in the sphenoid bone behind the optic chiasm called the
|
sella turcica
|
|
|
Extensions of the fibro-elastic capsule of dura mater that surrounds the pituitary gland consist almost entirely of
|
reticular fibers
|
|
|
The ... consistutes about three-fourths of the pituitary.
|
pars distalis
|
|
|
The parenchyma is arranged in the anastomosing cord pattern and is lined up with
|
sinusoidal capillaries
|
|
|
The two main parenchymal cell types in the pars distalis are
|
chromophobes and chromophils
|
|
|
4 characteristics of chromophobes
|
1) smaller than chromophils
2) lack secretory granules so cytoplasm does not stain 3) considered to be degranulated chromophils 4) cells tend to be located in clusters away from the capillaries |
|
|
3 characteristics of chromophils
|
1) tend to lie at the surface of the cell cords, next to capillaries
2) have granules which stain intensely 3) hormone-secreting cells of the adenohypophysis |
|
|
2 types of chromophils
|
1) acidophils (35% of all cells in pars distalis) - produce growth hormone and prolactin
2) basophils (15% of all cells in pars distalis) - stain with basic dyes and PAS+. Produce FSH, LH, TSH, ACTH. |
|
|
The primary and secondary capillary plexuses in the hypophyseal portal system are made up of
|
sinusoidal capillaries
|
|
|
All blood in the pars distalis pass through
|
the hypophyseal portal system
|
|
|
Purpose of the hypophyseal portal system
|
transport hormones from hypothalamus to the adenohypophysis
|
|
|
Flow of blood through hypophyseal portal system
|
Superior hypophyseal artery->primary capillary plexus->portal veins->secondary capillary plexus
|
|
|
The hypothalamus contains short neurosecretory neurons that
|
release small peptide products into the primary capillary plexus, which get to the pars distalis via the hypophyseal portal system and control hormone release from acidophils and basophils. Controlled by negative feedback and CNS input.
|
|
|
Two types of small peptide hormones synthesized in the neuron cell bodies in the hypothalamic nuclei
|
1) Releasing hormones (4)
2) Inhibiting hormones (2) |
|
|
Releasing hormones are (4)
|
1) GnRH - stimulates gonadotrophs to release FSH and LH
2) CRH - stimulates corticotrophs to release ACTH 3) TRH - stimulates thyrotrophs to release TSH 4) GHRH (somatotropin) - stimulates somatotrophs to release GH |
|
|
Inhibiting hormones (2)
|
1) Somatostatin (GHIH) - inhibits growth hormone release from somatotrophs
2) Dopamine (PIH) - inhibits prolactin release from mammotrophs |
|
|
An ill-defined region consisting of a thin layer of cells and colloid cysts (Rathke's cysts) next to the neural lobe. Atrophies in adults.
|
Pars Intermedia
|
|
|
Ventral down-growth of the hypothalamus and is made up of nervois tissue
|
posterior pituitary = neurohypophysis
|
|
|
Neurohypophysis is divided into (3)
|
1) medial eminence
2) infundibulum 3) pars nervosa |
|
|
Cell bodies for these modified multipolar neurons are located in the
|
paraventricular nucleus and the supraoptic nucleus
|
|
|
Hypothalamo-hypophyseal tract consists of
|
unmyelinated axons of the neurohypophysis.
|
|
|
Herring bodies are 1)... and 2) can contain ...
|
1) large granule-filled dilations along the length of axons or the pars nervosa as well as at their termini
2) ADH and oxytocin |
|
|
Somatostatin works through what type of G protein receptor?
|
Gi
|
|
|
Oxytocin and ADH are what type of hormones?
|
Peptide - 9 amino acids. Each differ in only 2 amino acids.
|
|
|
Neurophysin I is packaged with 1) ... and neurophysin II is packaged with 2) ... Both also packaged with 3)...
|
1) oxytocin
2) ADH 3) ATP |
|
|
Pituicytes are
|
highly branched astrocytes whose processes surround and support the axons of the pars nervosa (occupy about 25% of the volume)
|
|
|
Synthesized mainly by cells of the paraventricuar nucleus, stimulates milk ejection by the mammary glands, increased by increased suckling, stimulates uterine smooth muscle contraction during copulation and childbirth
|
oxytocin
|
|
|
synthesized mainly by the cells in the supraoptic nucleus, stimulates water resorption by the renal medullary collecting ducts
|
arginine vasopressin (AVP) = antidiuretic hormone (ADH)
|
|
|
2 most common types and causes of diabetes insipidus?
|
1) Central diabetes insipidus caused by ADH deficiency
2) Nephrogenic diabetes insipidus caused by an insensitivity of the kidneys to ADH. |
|
|
Glycoprotein hormones (stain with PAS) release from the pars distalis
|
TSH, FSH(female), FSH(male), LH(female), LH(male)
|
|
|
Source and target of TSH
|
S: thyrotroph (basophil)
T: follicular cells of thyroid |
|
|
Source and target of FSH (female)
|
S: gonadotroph (basophil)
T: follicular cells of ovary |
|
|
Source and target of FSH (male)
|
S: gonadotroph (basophil)
T: Sertoli cells of testis |
|
|
Source and target of LH (female)
|
S: gonadotroph (basophil)
T: Thecal, follicular, and luteal cells of ovary |
|
|
Source and target of FSH (male)
|
S: gonadotroph (basophil)
T: Leydig cells of testis |
|
|
Principle actions of TSH
|
Promotes thyroid growthl stimulates synthesis and liberation of T4 and T3 hormones; fatty acid release from fat cells
|
|
|
Principle actions of FSH (female)
|
Stimulates development and maturation of secondary ovarian follicles and their secretion of 17-beta estradiol
|
|
|
Principle actions of FSH (male)
|
Stimulates Sertoli cells to produce androgen binding protein
|
|
|
Principle actions of LH (female)
|
Elicits ovulation of Graafian follicle; promotes development of corpus luteum and progesterone secretion
|
|
|
Principle actions of LH (male)
|
Helps maintain Leydig cells of testis and stimulates their secretion of testosterone
|
|
|
Disorders of TSH
|
(+) Hyperthyroidism
(-) Hypothyroidism |
|
|
DNES is also referred to as
|
APUD
|
|
|
Small amount of RER, a supranuclear Golgi complex, and an accumulation of 100 to 400 nm secretory granules in their bases are characteristics of
|
polypeptide-secreting cells
|
|
|
APUD cells characteristically concentrate what in their cytoplasm?
|
important bioactive amines such as epinephrine, norepinephrine, and serotonin
|
|
|
Most APID cells are unicellular glands scattered among other epithelial cells, derived from
|
neural crest
|
|
|
True or False. Some DNES polypeptides have paracrine effects while other enter the bloodstream and have endocrine effects
|
True
|
|
|
Large populations of cells that secrete hormone-like substances, located in the enteric canal are
|
enteroendocrone cells
|
|
|
Three major digestive hormones secreted into the blood by enteroendocrine cells
|
1) Gastrin - promotes secretion of gastric juice, increases gastric motility, and promotes growth of gastric mucosa
2) Secretin - stimulates secretion of pancreatic juice and bile that are rich in HCO3-. 3) Cholecystokinin (CCK) - stimulates secretion of pancreatic juice rich in digestive enzymes, causes ejection of bile from the gallbladder and opening of the sphincter of Oddi, and induces satiety. |
|
|
Other gut-hormones (6)
|
1-3) motilin, substance P, bombesin - stimulate motility of intestines
4) Vasoactive intestinal peptide (VIP) - stimulates secretion of ions and water by the intestines and inhibits gastric acid secretion 5) gastrin-releasing peptide - stimulates release of gastrin 6) somatostatin - inhibits gastrin release |
|
|
Thyroid follicles are the main structural and function units of the thyroid gland and are made up of two main cell types
|
Follicular and parafollicular cells.
|
|
|
Thyroid gland is notable for its storage of reserve secretion called the
|
semifluid colloid which fills the lumen of the follicle. Stains acidophilic, consists mainly of the glycoprotein thyroglobulin.
|
|
|
True or False. Taller follicular cells are more active.
|
True
|
|
|
Function activity of follicular cells is stimulated by
|
TSH (a tropic hormone) from the anterior pituitary and by sympathetic nerves
|
|
|
3 factors can cause increased TSH
|
1) low environmental temperatures
2) puberty 3) pregnancy |
|
|
2 factors can cause decreased TSH
|
1) emotional stress
2) systemic stress such as trauma, heat, hemorrhage |
|
|
True or false. The production of thyroid hormones by the follicular cells involves an exocrine and endocrine part.
|
True
|
|
|
Thyroglobulin is produced from
|
amino acids entering the follicular cells (based on tyrosine).
|
|
|
Iodide enters the cell from vasculature via
|
iodide pump in the cell's basal plasma membrane. Iodide is oxidized by peroxidase and then transferred to the cell's apex. TSH also stimulates iodide uptake.
|
|
|
Iodination of tyrosine residues in the thyroglobulin occurs where?
|
at the microvillus-colloid interface
|
|
|
TSH causes
|
cells to pick up iodinated thyroglobulin->endosomes fuse with lysosomes->break down iodothyroglobulin into T3(10% but more active) and T4 (90%). 90% of T3 and T4 are bound with proteins in plasma. 1/2 is taken up in 6 days.
|
|
|
T3 and T4 act to
|
increase basal metabolic rate, promote cell growth, increase heart rate, raise body temperature, and generally enhance all energy-requiring cell functions.
|
|
|
Sequential steps of thyroid hormone synthesis (6)
|
1) synthesis and secretion of thyroglobulin
2) uptake and concentration of iodide from the blood, oxidation to iodine, and release into the colloid 3) iodination of thyroglobulin in the colloid 4) formation of T3 and T4 hormones in the colloid by oxidative coupling reactions 5) resorption of colloid by receptor-mediated endocytosis 6) release of T4 and T3 from the cell into the circulation |
|
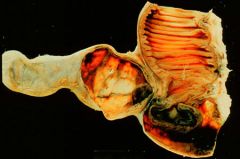
Parafollicular cells are derived from
|
neural crest
|
|
|
Parafollicular cells are
|
large, pale-staining cells which lie singly or in clusters between the follicular cells and their basal lamina; they do not reach the lumen of the follicle. 2-3 times larger than follicular cells, but only account for about 0.1% of the epithelium.
Neural crest derivatives and are APUD cells |
|
|
Parafollicular cells secrete
|
calcitonin (peptide hormone) in response to high blood calcium.
|
|
|
Calcitonin inhibits
|
bone resorption by inhibiting osteoclasts, thereby lowering blood calcium. Negligible physiologic effect under normal conditions in humans.
|
|
|
3rd pharyngeal pouch becomes 1) ... and 4th pharyngeal pouch becomes 2) ...
|
1) inferior parathyroid glands
2) superior parathyroid glands |
|
|
Broad, irregular ... channel the parenchymal cells of the parathyroids
|
fenestrated capillaries
|
|
|
Most abundant parenchymal cell in the parathyroids
|
chief cell, arranged in cords. Pale-staining cytoplasm, vesicular nuclei.
|
|
|
Chief cells secrete
|
parathyroid hormone (peptide hormone) - increases blood calcium
|
|
|
Parathyroid hormone acts at three locations
|
1) bone - PTH increases bone resorption
2) kidneys - PTH increases phosphate excretion and calcium reabsorption and causes activation of a vitamin D precursor 3) Intestines - PTH causes increased absorption of calcium by the intestinal mucosa. |
|
|
These cells are larger and less numerous than chief cells. Very acidophilic due to large amounts of mitochondria, lack secretory granules. Nucleus is small and darkly staining.
|
oxyphil cells - rare before puberty, increase in number in early adulthood
|
|
|
Tetany
|
hyperexcitability and spastic contraction of skeletal muscle
|
|
|
Removal of parathyroid glands cause
|
hypocalcemia, increased excitability of nervous tissue, including paresthesia and attacks of tetany or epilepsy. Administer PTH to correct.
|
|
|
Pyramid-shaped gland which sits on the right kidney
|
Right adrenal gland
|
|
|
Crescent-shaped gland that lies along the medial border of the left kidney from the hilus to the superior pole
|
Left adrenal gland
|
|
|
True or False. The adrenal glands are retroperitoneal, located behind the peritoneum and is surrounded by a connective tissue capsule containing large amounts of adipose tissue.
|
True
|
|
|
2 divisions of the adrenal glands
|
1) cortex - from mesoderm
2) medulla - from neural crest |
|
|
The accounts for 80-90% of the adrenal gland
|
Cortex
|
|
|
Adrenal cortex secretes what three steriod hormones (derived from cholesterol)?
|
1) mineralocorticoids
2) glucocorticoids 3) androgens |
|
|
Adrenal cortex is subdivided into three concentric layers
|
1) Zona glomerulosa (13-15%)
2) Zona fasciculata (78-80%) 3) Zona reticularis (7%) |
|
|
This layer of the adrenal cortex has cells arranged in clusters (some lipid droplets), and secrets mineralocorticoids (eg aldosterone)
|
Zona glomerulosa (13-15%)
|
|
|
Synthesis of mineralocorticoids is stimulated mainly by
|
angiotensin II
|
|
|
Mineralocorticoids function in
|
osmotic balance and blood pressure regulation by affecting the function of the renal tubules.
|
|
|
Dehydration or sodium deficiency ultimately causes the kidney to release
|
renin
|
|
|
Renin catalyzes
|
conversion of angiotensinogen to angiotensin I (inactive)
|
|
|
Angiotensin I (inactive) is converted to angiotensin II (active) by
|
angiotensin converting enzyme (ACE).
|
|
|
ACE is released by
|
pulmonary capillary endothelial cells
|
|
|
Angiotensin II acts on the
|
zona glomerulosa of the adrenal cortex to release aldosterone, which counteracts dehydration.
|
|
|
True or False. Angiotensin II is a powerful vasoconstrictor.
|
True
|
|
|
True or False. ANP inhibits aldosterone release from the zona glomerulosa.
|
True
|
|
|
This part of the adrenal cortex consists of cells arranged in cords or plates with sinusoidal capillaries running between them (many large lipid droplets in their cytoplasm).
|
Zona Fasciculata (78-80%). Cells called spongiocytes.
|
|
|
This portion of the adrenal cortex has cells which are irregularly arranged meshwork of cuboidal cells (fewer lipid droplets than spongiocytes). Produces androgens (dehydroepiandrosterone[DHEA]) and some androsterenedione. Both hormones are weak masculinizing hormones with negligible effects under normal conditions.
|
Zona reticularis (7%). Overproduction of these androgens in women can lead to masculinization.
|
|
|
The zona fasciculata and zona reticularis both produce
|
glucocorticoids (eg cortisol and corticosterone)
|
|
|
ACTH from the anterior pituitary stimulates
|
the zona fasciculata and zona reticularis to secrete glucocorticoids, which raise blood glucose.
|
|
|
This portion of the adrenal glands is nonessential for life but is an important regulator of stress. It is the central portion of the gland and is completely invested by the cortex.
|
Adrenal medula
|
|
|
The adrenal medulla contains two major cell types
|
1) chromaffin cells
2) ganglion cells |
|
|
The main parenchymal cells of the adrenal medulla are the
|
chromaffin cells
|
|
|
Chromaffin cells contain granules that stain intensely with chromaffin salts which indicate
|
that the cells contain catecholamines
|
|
|
These cells are modified postganglionic sympathetic neurons which have lost their axons and dendrites, and have an epithelioid appearance
|
Chromaffin cells
|
|
|
These cells are part of the DNES and migrate from the celiac ganglion
|
chromaffin cells
|
|
|
These cells are innervated by preganglionic sympathetic fibers, use ACh as the neurotransmitter
|
chromaffin cells
|
|
|
Chromaffin cells make 1) 85% ... and 2) 15% ...
|
1) epinephrine
2) norepinephrine |
|
|
Effects of epinephrine
|
1) increases blood glucose
2) increases alertness 3) increases cardiac output 4) increases heart rate In preparation for fight-or-flight, plasma levels may increases as much as 300 times. |
|
|
Epinephrine is stored in
|
clear granules (characteristic of cells producing epinephrine)
|
|
|
Effects of norepinephrine
|
1) increases blood pressure by vasoconstriction
Stored in dense-core granules (characteristic of cells producing norepinephrine) |
|
|
These cells of the adrenal medulla exhibit typical morphologic characteristics of autonomic ganglion cells, and are scattered throughout the connective tissue and are vasomotor
|
ganglion cells
|
|
|
These (2) blood supplies to the adrenal glands run between the capsule and the medulla and drain into the medullary veins
|
sinusoidal capillaries and cortical arterioles
|
|
|
This enzyme converts norepinephrine to epinephrine, is activated by cortical steroids
|
Phenylethanolamine-N-methyl transferase (PNMT)
|
|
|
Chromaffin cells supplied by the sinusoidal capillaries will receive blood with high levels of cortical steroids and therefore will produce
|
epinephrine (most of the adrenal medulla product)
|
|
|
Chromaffin cells supplied by the medullary arterioles will receive very little cortical steroids and will therefore produce
|
norepinephrine
|
|
|
Stress, provoked by psychological, environmental, or physiologic stressor, leads to throughts and emotions that influence both the CNS and the immune system, activating a bidirectional circuit between the two, and occurs via the
|
hypothalamic-pituitary-adrenal (HPA) axis. This initiates a cascade of reactions throughout the individual
|
|
|
This is produced by the hypothalamus, stimulates the synthesis and processing of proopiomelanocortin, with resulting release of proopiomelanocortin peptides that include ACTH from the anterior pituitary.
|
Corticotropin-releasing factor (CRF)
|
|
|
ACTH binds to the melanocortin-2 receptor in the adrenal gland and stimulates
|
cholesterol-derived synthesis of adrenal steroid hormones
|
|
|
Glucocorticoids released into the systemic circulation exert negative feedback inhibition of
|
CRF and ACTH release from the hypothalamus and pituitary, respectively.
|
|
|
Major stress hormones are
|
Epinephrine, norepinephine, and corticosteroids
|
|
|
Prolonged duration and increased magnitude of activation of the neuroendocrine response can lead to
|
erosion of lean body mass and tissue injury
|
|
|
Mineralocorticoids control
|
water and electrolyte content of the body fluids
|
|
|
These mobilize and increase production of glycose so that it is available as an immediate energy source
|
Glucocorticoids
|
|
|
Catecholamines stimulate
|
the cardiovascular and respiratory systems and the general metabolic activity of the body. They also assist the glycocorticoids in raising the blood glucose level and assist the sympathetic nervous system.
|
|
|
True or False. Elevated corticosteroid have profound immunosuppresive effects.
|
True
|
|
|
The physiolocal role of the fetal zone of the adrenals in fetal life is to
|
synthesize estrogen precursors, which are converted in the placenta to estrogens.
|
|
|
Islets of Langerhans for part of the DNES and derived from
|
endoderm
|
|
|
Four main types of cells compose the parenchyma of each islet
|
1) beta cells
2) alpha cells 3) delta cells 4) F, or pancreatic peptide (PP), cells. |
|
|
Islets of Langerhans: Beta cells
|
Scattered throughout the islets, but concentrated in the center (70% of total cells). Produce insulin. Malfunction causes diabetes mellitus
|
|
|
Islets of Langerhans: Alpha cells
|
located mostly along the periphery (15-20% of total islets). Produce glucagon which acts mainly on hepatocytes.
|
|
|
Islets of Langerhans: Delta cells
|
produce somatostatin (made in many places, works through Gi) which inhibits alpha and beta cells. Located along periphery (5-10% of total islet)
|
|
|
Islets of Langerhans: F (PP) cells
|
produce pancreatic polypeptide, which inhibits pancreatic exocrine secretion of enzymes and bicarbonate. Located mostly along the periphery (1% of total islet)
|
|
|
Exocrine product of follicular cells
|
thyroglobulin
|
|
|
Endocrine product of follicular cells
|
T3 and T4
|
|
|
Secretions of this endocrine gland are influenced by light and dark periods of the day
|
pineal gland
|
|
|
Interstitium of the pineal gland contains calcified concretions known as
|
corpora arenacea (brain sand) - seen on x-ray. Composed primarily of calcium carbonates and phosphates within an organic matrix.
|
|
|
Two types of parenchyma cells in the pineal gland
|
1) pinealocytes - pale-staining, lobulated nuclei.
2) astrocytes - occur in the perivascular areas and between the clusters of pinealocytes. Elongated nuclei and stain more deeply than pinealocytes. |
|
|
Pinealocytes produce
|
serotonin during the day and melatonin at night.
|
|
|
Circadian rhythms are controlled by the periodic release of
|
norepinephrine from postganglionic sympathetic fibers, which in turn is controlled by light perceived by the retina.
|
|
|
The pineal contains what enzyme which has a role in the synthesis of melatinin?
|
hydroxyindole-O-methyltransferase (HIOMT)
|
|
|
Melatonin is primarily controlled via the
|
hypothalamic suprachiasmatic nuclei (SCN)
|
|
|
During the day, impulses from the SCN
|
suppress the synthesis of melatonin from seratonin.
|
|
|
True or False. Practically no storage of melatonin takes place and therefore, secretion occurs at the time of synthesis.
|
True
|
|
|
Considered to be the "master gland"
|
pineal gland
|
|
|
Thyroid gland hormones
|
T4 and T3 (follicular cells), Calcitonin (parafollicular cells)
|
|
|
Parathyroid gland hormone
|
PTH (chief cells)
|
|
|
Adrenal cortex hormones
|
Mineralocorticoids(aldosterone and deoxycorticosterone) (cells of zona glomerulosa)
Glycocorticoids (cortisol and corticosterone)(cells of zona fasciculata and zona reticularis) Gonadocorticoids (dehydroepiandrosterone)(cells of zona reticularis) |
|
|
Hormones or adrenal medulla
|
epinephrine and norepinephrine (chromaffin cells)
|
|
|
Hormones or Pancreatic Islets of Langerhans
|
glucagon (alpha cells)
insulin (beta cells) somatostatin (delta cells) pancreatic polypeptide (PP, or F cells) |
|
|
Hormones of the pineal gland
|
Melatonin (at night) (pinealocytes)
Seratonon (at day) (pinealocytes) |
|
|
Target cells of calcitonin
|
osteoclasts, epithelial cells of kidney tubules
|
|
|
Target cells of PTH
|
osteoblasts, small intestine, PCT of kidneys
|
|
|
Target cells of aldosterone and deoxycorticosterone
|
DCT epithelial cells, gastric mucosa, salivary/sweat ducts
|
|
|
Target cells of somatostatin
|
pancreatic endocrine cells
|
|
|
Target cells of pancreatic polypeptide
|
pancreatic exocrine cells
|
|
|
Target cells of melatonin and seratonin
|
hypothalamus neurosecretory cells, pitutary gonadotrophs, gonads
|
|
|
Action of T4, and T3
|
(+) BMR, heat production, O2 comsumption, protein synthesis, glucose/fatty acid use, cholesterol excretion, skeletal growth, nervous system development
|
|
|
Action of calcitonin
|
negligible under normal conditions, (-) blood calcium/phosphates, bone resorption by osteoclasts, calcium/phosphate reabsorption by kidneys
|
|
|
Action of PTH
|
(+) blood calcium/magnesium, bone resorption by osteoclasts, calcium reabsorption by kidneys/gut, phosphate excretion by kidneys, calcitrol (Vitamin D) formation, (-) blood phosphate
|
|
|
Action of aldosterone and deoxycorticosterone
|
(+) reabsorption of sodium, hydrogen/potassium secretion in DCT of kidneys, gastric mucosa, salivary/sweat glands, controls body fluid volume, [electrolyte]
|
|
|
Action of cortisol and corticosterone
|
(+) gluconeogenesis, blood glucose, protein/lipid/carb metabolism, anti-inflammatory, stress resistance (-) immune response
|
|
|
Action of dehydroepiandrosterone
|
negligible under normal conditions
|
|
|
Action of epinephrine and norepinephrine
|
E: (+) blood glucose, cardiac output, BMR, N: (+) BP by vasoconstriction
|
|
|
Action of Glucagon
|
(+) blood glucose, gluconeogenesis from amino acids, glycogenolysis
|
|
|
Action of Insulin
|
(-) blood glucose, glucose uptake, protein synthesis, lipid synthesis in fat cells
|
|
|
Action of somatostatin
|
(-) glucagon/insulin release
|
|
|
Action of pancreatic polypeptide
|
(-) somatostatin release, pancreatic exocrine release, gallbladder contraction
|
|
|
Action of melatonin and seratonin
|
photoperiodically regulates circadian rhythms, regulates steroidogenic activity of gonads (menstrual cycle), (-) GnRH release from hypothalamus
|
|
|
Disorders involving T4 and T3
|
(+) Graves' disease, (-) Myxedema (adults), Cretinism (children)
|
|
|
Disorders involving PTH
|
(+) Osteitis fibrosa, (-) Hypocalcemia w/muscle tetany
|
|
|
Disorders involving Aldosterone, Deoxycorticosterone
|
(+) Cushing's Disease, (-) Addison's Disease (sodium loss)
|
|
|
Disorders involving Cortisol, Corticosterone
|
(-) Addison's Disease (low glucose)
|
|
|
Disorders involving Dehydroepiandrosterone
|
(+) female masculinization
|
|
|
Disorders involving Epinephrine, Norepinephrine
|
(+) Pheochromocytoma
|
|
|
Disorders involving Insulin
|
(+) Insulin Shock, (-) Diabetes mellitus
|
|
|
Disorders involving Melatonin (night), Serotonin (day)
|
(+) delayed sexual development, (-) premature sexual development
|
|
|
Steroid hormones (adrenal gland)
|
1) glucocorticoids
2) mineralocorticoids 3) androgens |
|
|
Protein sex hormones (gonadotropins)
|
1) follicle stimulating hormone
2) luteinizing hormone 3) human chorionic gonadotropin |
|
|
Steroid hormones (gonadal)
|
1) estrogen
2) progesterone 3) testosterone |
|
|
Types of hormones (7)
|
1) Peptide
2) Thyroid 3) Catecholamine, serotonin & melatonin 4) Acetylcholine 5) Histamine 6) Nitric Oxide 7) Eicosanoid |
|
|
Tissues that produce hormones include (8)
|
1) hypothalamus
2) anterior and posterior pituitary 3) adrenal cortex and medulla 4) gonads 5) thyroid and parathyroid glands 6) gastrointestinal tract cells 7) pancreas 8) heart |
|
|
The neuroendocrine system is centered on
|
1) hypothalamus
2) pituitary gland 3) adrenal gland |
|
|
The neuroendocrine system is responsible for regulating
|
hormone release and function throughout the body
|
|
|
The hypothalamus is a region of the brain responsible for
|
maintaining homeostatic mechanisms in the body
|
|
|
Pituitary gland receives input from
|
hypothalamus
|
|
|
Hormones which are amino acid derivatives
|
T4, T3, catecholamines, serotonin, melatonin, histamine
|
|
|
Homones which are arachidonic acid derivatives
|
Eicosanoids
|
|
|
Hormones which are cholesterol derivatives
|
Steroids
|
|
|
Nitric oxide is produced by nitric oxide synthase using what substrate?
|
L-arginine, NADPH, and O2
|
|
|
Overproduction of a particular hormone commonly results from
|
tumor formation
|
|
|
Underproduction of a particular hormone can be caused by
|
auto-immune disorders, genetic defects or cancer.
|
|
|
Target cell insensitivity is commonly caused by
|
a lack of functional receptors due to genetic defects, autoimmune disorders or overstimulation and downregulation of receptors
|
|
|
Synthesis pathway of peptide hormones
|
preprohormone(synthesized in rER)->prohormone (cleaved in rER)->hormone (packaged in Golgi with required processing proteins in "immature" secretory granules)
|
|
|
Processing in "immature" secretory granules includes
|
proteolytic cutting the prohormone at the C-terminal side of paired-basic residues (ie lysine and arginine) (requires acidic environment pH~4 generated by H+ATPases)
Resulting peptide hormines then have the C-terminal lysine/arginine removed and are amidated, which makes them more biologically active and helps preserve their halflife. |
|
|
Peptide hormones have short half-lives (minutes) because they are
|
rapidly degraded by serum and cell surface proteases and taken up by cells through receptor mediated endocytosis and degraded by lysosomal proteases
|
|
|
Regulation of ACTH (peptide hormone) release is through (3)
|
1) Feedback inhibition by cortisol at the anterior pituitary (direct inhibition of ACTH release and production)
2) Feedback inhibition by cortisol at the hypothalamus (inhibits release of CRH) 3) Negative inhibition by dopamine (inhibits release of ACTH from anterior pituitary) |
|
|
Peptide prohormones are cleaved by
|
processing enzymes called Prohormone Convertases (PC1/3, PC2)
|
|
|
Differential processing of prohormones is based on
|
different cleavage locations of PC1 and PC2. PC2 cleaves in more places. Other factors include conditions such as stress.
|
|
|
Difference between Cushing's syndrome and Cushing's disease?
|
Both are hypercortisolism.
Cushing's disease is caused by ACTH secreting tumors in the pituitary (most common form of Cushing's). Females >> males Cushing's syndrome is an adenoma of the adrenal cortex, releasing excess cortisol. |
|
|
Treatment of Cushing's syndrome
|
Ketoconazole or metyrapone
|
|
|
Treatment of Cushing's disease
|
surgical remove of ACTH secreting tumor
|
|
|
Diabetes Insipidus
|
1/10,000. Polyuria, polydypsia
|
|
|
Thyroid hormones
|
DIT + DIT -> T4
DIT + MIT -> T3 |
|
|
Five steps of thyroid hormone production
|
1) Thyroglobulin synthesis and processing in the ER and Golgi
2) Thyroglobulin is packaged and moved to colloid space where it is iodinated 3) TSH binds, iodinated TG moves back to the cell, fuses with lysosomes, proteases cleave the peptide bonds to form T3 and T4. 4) Release of T3 & T4 regulated by TSH - binds to receptors on follicular cells and increases cAMP. Secretion occurs rapidly after T3 & T4 production 5) T4 goes to T3 in peripheral tissue. Liver degenerates T4 and T3. Can also be conjugated with glucuronides and sulfates (bile). |
|
|
Approximately how much plasma T3 is produced by the thyroid?
|
20%. The other 80% comes from deiodination of T4.
|
|
|
Thyroid hormones are primarily eliminated by the
|
kidneys. However approximately 20% of T4 reaches the colon.
|
|
|
TSH is produced by the
|
anterior pituitary
|
|
|
TRH is produced by the
|
hypothalamus
|
|
|
T3 & T4 negatively feeds back to the
|
hypothalamus (inhibits TRH production)
|
|
|
What do thyroid hormones do in the fetus?
|
Required for skeletal and CNS growth
|
|
|
What do thyroid hormones do in adult?
|
effect BMR as well as protein, lipid and carbohydrate metabolism.
|
|
|
T4 is converted to T3 by
|
5'-iodinase
|
|
|
Cause of hypothyroidism?
|
Insufficient circulation of free T3 or T4
|
|
|
Symptoms of hypothyroidism (3)
|
1) Lowered BMR
2) Diastolic hypertension 3) Goiter |
|
|
Treatment for hypothyroidism?
|
Levothyroxine
|
|
|
Cause of hyperthyroidism?
|
Autoimmune - antibodies to TSH receptor
|
|
|
Symptoms of hyperthyroidism? (3)
|
1) Increased metabolic rate
2) Exophthalmos (protruding eyes) 3) Goiter |
|
|
Treatment of hyperthyroidism? (2)
|
1) Surgical resection of the thyroid gland
2) 131-I to destroy hyperfunctioning thyroid acinar cell population |
|
|
Sequence of hypothyroidism
|
decreased T3 and T4 production -> increased secretion of TSH from pituitary -> increased levels of TSH cause thyroid to increase T3 and T4 production -> enlarges thyroid to produce more hormone
|
|
|
Graves disease is
|
hyperthyroidism
|
|
|
Hyperthyroidism is caused by
|
continuous activation of TSH receptors by antibodies to TSH receptors. Increases T4 and T3 production which causes the thyroid to increase in size.
|
|
|
Function of epinephrine
|
Fight or flight response
|
|
|
Function of norepinephrine (2)
|
1) fight or flight response
2) attention |
|
|
Function of dopamine (2)
|
1) focus
2) inhibits prolactin from pituitary |
|
|
Rate limiting step in the synthesis of catecholamines
|
tyrosine hydroxylase
|
|
|
Enzyme for Tyrosine->L-DOPA
|
tyrosine hydroxylase
|
|
|
Enzyme for L-DOPA -> dopamine
|
L-Dopa decarboxylase
|
|
|
Enzyme for dopamine -> norepinephrine
|
dopamine-beta-hydroxylase
|
|
|
Enzyme for norepinephrine -> epinephrine
|
phenylethanolamine-n-methyl transferase
|
|
|
Degradation of catecholamines is done by what enzymes?
|
COMT and MAO
|
|
|
Synthesis location of epinephrine?
|
Adrenal medulla, where it constitutes 80% of the catecholamines
|
|
|
Synthesis location of norepinephrine? (3)
|
1) Sympathetic nerves
2) Adrenal medulla 3) CNS |
|
|
Synthesis location of dopamine?
|
CNS (CANNOT cross the blood-brain barrier)
|
|
|
True or False. L-DOPA can cross the blood-brain barrier.
|
True
|
|
|
Pheochromocytoma is
|
a tumor of in the adrenal medulla
|
|
|
Symptoms of pheochromocytomas
|
hypertension and tachycardia caused by excessive production of catecholamines
|
|
|
Treatment of pheochromocytomas
|
surgery, radiation, chemotherapy
|
|
|
Serotonin plays a role in (5)
|
1) mood
2) sleep 3) emesis (vomiting) 4) sexuality 5) appetite |
|
|
Disorders involving serotonin (4)
|
1) depression
2) migraine 3) bipolar disorder 4) anxiety |
|
|
Melatonin is believed to be responsible for
|
regulating sleep/wake cycles
|
|
|
Serotonin and melatonin are produced in the
|
pineal gland
|
|
|
True or False. Serotonin is secreted by cells in the small intestine, where it serves as a potent vasoconstrictor and smooth muscle stimulator
|
True
|
|
|
Serotonin and melatonin levels undergo cyclic variations in phase with
|
circadian rhythms
|
|
|
Serotonin and melatonin are synthesized from
|
tryptophan.
|
|
|
Tryptophan is first transported into the cell and acted on by ... which is the rate limiting step for serotonin and melatonin biosynthesis
|
tryptophan hydroxylase
|
|
|
Synthesis of serotonin
|
tryptophan->5-hydroxytryptophan->serotonin
Enzymes: 1) tryptophan hydroxylase 2) 5-hydroxytryptophan decarboxylase |
|
|
Synthesis of melatonin
|
serotonin->n-acetylserotonin->melatonin
Enzymes 1) serotonin n-acetyltransferase 2) hydroxy indole o-methyltransferase (HIOMT) |
|
|
True or False. Serotonin must be removed or it will continue to elicit a response.
|
True
|
|
|
Two mechanism for serotonin removal
|
1) Broken down by MAO
2) Reuptake by sodium depended transport protein (vesicular transport) |
|
|
Selective serotonin reuptake inhibitors (2)
|
1) fluoxetine (Prozac)
2) paroxetine (Paxil) |
|
|
Treatment for depression (2)
|
1) Inhibit MAO
2) Prevent removal of NT's from synaptic clefts allowing them to remain longer |
|
|
Cocaine inhibits
|
reuptake of dopamine, norepinephrine, and serotonin.
|
|
|
Inhibitors of tryptophan hydroxylase
|
p-chlorophenylalanine
alpha-propyldopacetamide |
|
|
Inhibitor of MAO
|
Iproniazid
|
|
|
Vesicular monoamine transport blocker
|
reserpine
|
|
|
Cause of Hartnup's disease
|
Genetic metabolic disorder (defective absorption of the amino acid tryptophan)
|
|
|
Symptoms of Hartnup's disease
|
Early onset of
1) photosensitive dermatitis 2) intermittent neurologic symptoms 3) Blue diaper syndrome (indicanuria) |
|
|
Treatment of Hartnup's disease
|
1)high protein diet
2)add di-peptide tryptophan-tryptophan, which is transported by a di-peptide transporter |
|
|
The acetylcholinesterase of the end plate is attached to
|
the basal lamina in the synaptic space
|
|
|
True or False. 20% of release ACh is hydrolyzed by AChE before binding to the AChR.
|
True
|
|
|
ACh binding to AChR causes the cation-selective channel to open allowing entry to
|
Na+ and Ca++
|
|
|
Each vesicle in the motor nerve terminal contains a quantum of about
|
10,000 molecules of ACh
|
|
|
Enzyme which synthesizes ACh from Ch
|
choline acetyltransferase
|
|
|
ACh is concentrated in synaptic vesicles by
|
H+ dependent ACh transporter
|
|
|
Steps of ACh degradation and reuptake
|
1) Degraded to Ch and acetate
2) Ch taken up by nerve terminal Na+ dependent active transport 3) Ch->ACh 4) ACh into vesicles |
|
|
Myasthenia Gravis is caused by
|
autoimmune disease (acetylcholine receptors are target of inflammation)
|
|
|
Treatment of myasthenia gravis
|
pyridostigmine, inhibits AChE so there is an increase in ACh in the neuromuscular junction
|
|
|
Common symptoms of myasthenia gravis
|
1) double vision
2) droopy eyelids 3) difficulty swallowing 4) generalized weakness |
|
|
Organophosphates do what to AChE?
|
Inhibit
|
|
|
Prophylactic treatment of organophosphate exposure
|
Pyridostigmine or other competitve inhibitors of AChE
|
|
|
Post-Exposure treatment of organophosphate exposure
|
Atropine, Diazepam, Pralidoxime (2-PAM)
|
|
|
Function of histamine
|
potent vasodilator. Released locally at sites of trauma, inflammation, and allergic reaction. It causes enlargement of blood capillaries and can cause lowering of the blood pressure which can lead to shock.
|
|
|
Four histamine receptors
|
H1-4
|
|
|
H1 histamine receptor
|
found on smooth muscle, endothelium, and CNS tissue. Causes vasodilation, bronchoconstriction, smooth muscle activation, and separation of endothelial cells (responsible for hives), and pain and itching due to insect stings; the primary receptors involved in allergic thinitis symptoms and motion sickness. Stimulates gastric acid secretion in stomach.
|
|
|
H2 histamine receptor
|
located on parietal cells, which primarily regulate gastric acid secretion.
|
|
|
H3 histamine receptor
|
decreases neurotransmitter release: histamine, acetylcholine, norepinephrine, serotonin
|
|
|
H4 histamine receptor
|
unknown physiological role. Found primarily in the thymus, small intestine, spleen, and colon. It is also found on basophils and in the bone marrow.
|
|
|
Histamine synthesis
|
Histidine->histamine->stored in secretory vesicles inside until release by mast cells and basophils.
|
|
|
Histamine breakdown
|
done by histamine-N-methyltransferase and diamine oxidase. Histamine is taken up by a transporter.
|
|
|
Histamine responses
|
H1 receptor increases IP3 & DAG
1) smooth muscle, endothelium & brain 2) causes vasodilation, bronchoconstriction, smooth muscle activation 3) separation of endothelial cells (responsible for hives) 4) pain and itching due to insect stings 5) the primary receptors involved in allergic rhinitis symptoms and motion sickness |
|
|
Treatment of allergic reactions
|
Antihistamines
1) diphenhydramine (benadryl) - 1st generation 2) Fexofenadine (allegra) 3) Loratadine (Claritin - 2nd generation |
|
|
Overuse of nasal sprays may lead to
|
a rebound effect (rhinitis medicamentosa)
|
|
|
Diphenhydramine competitively antagonizes the effects of histamine of H1-receptors in (4)
|
1) GI tract
2) uterus 3) large blood vessels 4) bronchial muscle |
|
|
Blockade of H1-receptors also suppresses the formation of
|
edema, flare, and pruritus that result from histaminic activity
|
|
|
Nitric oxide synthesis
|
Arginine + O2 + NADPH + nitric oxide synthase -> NO + Citrulline
|
|
|
NO is broken down by
|
SOD and H2O2.
|
|
|
Effects of ACh and Bradykinin on NOS
|
stimulates
|
|
|
Effects of glucocorticoids on NOS
|
inhibits
|
|
|
Nitric oxide increases cGMP synthesis which
|
acts to inhibit platelet aggregation
|
|
|
Sildenafil (Viagra) and tadalafil (Cialis) are selective inhibitors of
|
cGMP specific phosphodiesterase type 5, which is responsible for degradation of cGMP in the corpus cavernosum.
|
|
|
Types of eicosanoids (4)
|
1) Thromboxanes
2) Prostacyclin (PGI2) 3) Leukotrienes 4) Prostaglandins |
|
|
Eicosanoids play a central role in
|
the inflammatory response
|
|
|
Eicosanoids are synthesized by all mammalian cells except
|
erythrocytes
|
|
|
Function of thromboxane
|
1) vasoconstrictor
2) induces platelet aggregation |
|
|
Function of prostacyclin (OGI2)
|
1) Vasodilator
2) Inhibits platelet aggregation |
|
|
Synthetic prostacyclin analogues
|
iloprost and cisaprost. Used as a vasodilator and to treat pulmonary hypertension
|
|
|
Production of prostacyclin is inhibited indirectly by
|
NSAIDS which inhibit COX1 and COX2
|
|
|
Function of leukotrienes
|
Chemotaxis
Inflammation Allergic reactions SRS-A (slow reacting substance of anaphylaxis = SM contraction) -mixure of leukotrienes (C4,D4,E4) -1000X more potent than histamine |
|
|
Leukotriene B4 recruits
|
neutrophils and eosinophils to the site of inflammation
|
|
|
Leukotrienes act principally on a subfamily of
|
G protein coupled receptors
|
|
|
Leukotriene receptor antagonists are used to treat
|
asthma (eg montelukast)
|
|
|
Examples of leukotrienes
|
LTA4, LTB4, LTC4. LTD4, LTE4, LTF4
|
|
|
Function of prostaglandins (4)
|
1) muscle constriction
2) mediate inflammation 3) calcium movement 4) hormone regulation |
|
|
Five stages to eicosanoids synthesis
|
phospholipid->arachidonic acid(occurs inside granules of mast cells)->stored in secretory vesicles.
Rapid degraded due to their own instability by oxidation of 15ahydroxyl group. |
|
|
Inhibition of Phospholipase A2 by
|
lipocortins (annexins)
|
|
|
Inhibition of prostaglandin endoperoxide synthase by
|
Aspirin, ibuprofen, acetaminophen
|
|
|
Inhibition of lipoxygenase or leukotriene receptors by
|
Zileuton, montelukast and zafirlukast
|
|
|
3 steroid hormone classifications
|
1) glucocorticoids
2) mineralocorticoids 3) androgens (DHEA) |
|
|
Glycocorticoids affect
|
intermediary metabolism
|
|
|
Mineralocorticoids affect
|
salt-retaining activity
|
|
|
Androgens (DHEA) affect
|
sex hormones
|
|
|
True or False. Steriods are not stored in vesicles like other hormones, but readily diffuse out of the cell as soon as they are made.
|
True
|
|
|
Steroids are degraded by
|
dehydrogenases in the hepatic and extrahepatic sites.
|
|
|
Two glucocorticoids
|
1) Cortisol
2) Corticosterone |
|
|
Made in the adrenal cortex, involved in response to stress, increases blood pressure, blood sugar levels, suppresses immune system.
|
Cortisol
|
|
|
Made in the zona glomerulosa of the adrenal cortex. Intermediate in the pathway from pregnenolone to aldosterone.
|
Corticosterone
|
|
|
Transcortin is
|
corticosteroid-binding globulin
|
|
|
Glucocorticoids inhibit
|
CRH, ACTH, and cortisol secretion
|
|
|
Effect of prolonged glucocorticoid administration (3)
|
1) suppression of CRH and ACTH release
2) atrophy of the zonae fasciculata and reticularis 3) suppressed HPA-axis fails to response to stress and stimulation |
|
|
Approximately half of the total daily cortisol output is secreted during
|
3rd - 8th hours of sleep
|
|
|
Symptoms of stress (2)
|
1) ACTH and cortisol increase within minutes
2) Abolish circadian periodicity if the stress is prolonged |
|
|
The stress response originates in the CNS by
|
increasing CRH secretion from the hypothalamus
|
|
|
Treatment for stress
|
1) Regular exercise
2) reassurance 3) biofeedback 4) counseling |
|
|
Principle mineralocorticoid is
|
aldosterone
|
|
|
Mineralocorticoids are produced in the
|
zona glomerulosa
|
|
|
Principle action of mineralocordicoids is
|
regulation of electrolytes
|
|
|
Renin-angiotensin system
|
1) Decreased blood flow to kidneys
2) Kidney secrete RENIN 3) RENIN reacts with ANGIOTENSINOGEN to produce ANGIOTENSIN I (weak vasoconstrictor) 4) ACE in the lungs convert ANGIOTENSIN I->ANGIOTENSIN II (strong vasoconstrictor) 5) ANGIOTENSIN II acts on the adrenal cortex to release ALDOSTERONE 6) ALDOSTERONE stimulates Na+/K+ ATPase 7) Na+ reabsorbed (along with water) 8) Increased blood volume, decreased urine volume 9) Increased blood pressure |
|
|
Release of renin by the kidney caused by (3)
|
1) sympathetic stimulation of beta1-adrenoceptors
2) renal artery hypotension 3) decreased sodium in the distal tubules |
|
|
Functions of angiotensin II (6)
|
1) Constricts resistance vessels (via ATII receptors) thereby increasing systemic vascular resistance and arterial pressure
2) Acts upon the adrenal cortex to release aldosterone, which in turn acts upon the kidneys to increase sodium and fluid retention 3) stimulates the release of ADH from the posterior pituitary which acts upon the kidneys to increase fluid retention 4) stimulates thirst centers within the brain 5) facilitates norepinephrine release from sympathetic nerve endings and inhibits norepinephrine reuptake by nerve endings, thereby enhancing sympathetic adrenergic function 6) Stimulates cardiac hypertrophy and vascular hypertrophy |
|
|
ACE inhibitors and AII receptor blockers are used to
|
decrease arterial pressure, ventricular afterload, blood volume and ventricular preload.
Reverses cardiac and vascular hypertrophy |
|
|
Generic term for any natural or synthetic compound, usually a steroid hormone, that stimulates or controls the development and maintenance of masculine characteristics in vertebrates by binding to androgen receptors
|
Androgen
|
|
|
True or False. Androgens are the original anabolic steroids.
|
True
|
|
|
The primary and most well-known androgen is
|
testosterone
|
|
|
4 adrenal androgens (produced by the adrenal cortex)
|
1) DHEA
2) DHEA sulfate 3) androstenedione 4) testosterone |
|
|
DHEA is a precursor of
|
natural estrogens
|
|
|
Androstenedione is produced by
|
testes, adrenal cortex, and ovaries. Metabolically converted to testosterone.
|
|
|
Steroid metabolite thought to act as the main regulator of gonadotropin secretion
|
Androstanediol
|
|
|
Breakdown product of androgens
|
Androsterone
|
|
|
A metabolite of testosterone that is actually more potent
|
dihydrotestosterone
|
|
|
Measurements of these are useful to detect excess adrenal activity as seen in adrenal cancer or hyperplasia, including certain forms of congenital adrenal hyperplasia
|
DHEA/DHEA sulfate
|
|
|
Mineralocorticoids are produced in the
|
zona glomerulosa
|
|
|
Glucocorticoids are produced in the
|
zona fasciculata
|
|
|
Androgens are produced in the
|
zona reticularis
|
|
|
Types of gonadotropins (3)
|
1) FSH
2) LH 3) hCG |
|
|
FSH is produced by the
|
pituitary gland
|
|
|
Functions of FSH (4)
|
1) Promotes and sustains the ovarian follicular growth in women
2) Promotes and sustains spermatogenesis in men 3) Stimulates the synthesis of its own receptor on granulosa and Sertoli cells 4) Stimulates aromatase activity inside granulosa cells |
|
|
Aromatase is an enzyme that converts
|
androgens into estrogens
|
|
|
FSH synthesis and secretion by the hypophysis is controlled by different regulators such as (3)
|
1) GnRH
2) ovarian estrogens 3) activin and inhibin |
|
|
LH is produced by the
|
hypophysis (pituitary)
|
|
|
Three main functions of LH
|
1) Promotes androgen synthesis in a) thecal cells of ovaries b) interstitial cells of testes
2) Induces ovulation 3) Maintains the corpus luteum during the menstrual cycle |
|
|
hCG is produced by
|
syncytiotrophoblast cells of the placenta
|
|
|
Functions of hCG (2)
|
1) Maintains corpus luteum of pregnancy (promotes progesterone secretion)
2) Anti-gonadotrophic effect (inhibits LH&FSH, Stimulates steroid secretion from fetal gonads) |
|
|
Prolactin (a lactotroph) is produced by (2)
|
1) pituitary
2) stromal cells of endometrium |
|
|
Functions of prolactin (2)
|
1) Breast development
2) Lactation |
|
|
Pituitary prolactin secretion is inhibited by 1) ... and stimulated by 2) ...
|
1) dopamine
2) TRH and VIP |
|
|
Steroid hormones (3)
|
1) esterogen
2) progesterone 3) testosterone |
|
|
Rate limiting step of steroid hormone synthesis
|
Conversion to pregnenolone by remove of 6 carbon frament (isocaproic acid)
|
|
|
Steroid hormone synthesis is controlled by
|
LH from the anterior pituitary
|
|
|
Maintains a high local concentration of testosterone in the vicinity of developing spermatozoa
|
androgen binding protein
|
|
|
Three types of estrogen steroids
|
1) Estrone (E1)
2) Estradiol (E2) 3) Estriol (E3) |
|
|
True or False. At equal concentrations, E2 > E1 > E3
|
True
|
|
|
Most common natural estrogen formulation
|
10% E1
10% E2 80% E3 |
|
|
Functions of estrogen (7)
|
1) Development of secondary sexual organs and features
2) Maturation of germ cells 3) Development of uterus 4) Maintenance of endometrium 5) Establish timing of ovulation 6) Maintenance of pregnancy 7) Mammary gland development and lactation |
|
|
Estrogen circulating in the blood can feedback to reduce circulating levels of
|
FSH and LH
|
|
|
Synthesis of estrogens occurs in
|
1) developing follicles in the ovaries, corpus luteum, and placenta
2) liver, adrenal glands, breasts |
|
|
Synthesis pathway of estrogen
|
cholesterol->androstenedione->estrone or estradiol
|
|
|
Androgens are produced by the thecal cells under the influence of
|
LH
|
|
|
Androgens are converted to E2 in the granulosa cells of the follicle through the enzyme
|
aromatase
|
|
|
E1 is produce in the
|
thecal cells, under the influence of LH
|
|
|
Very low concentrations of E3 are produce in
|
the liver, hydroxylation of E1 and E2
|
|
|
During pregnancy, E3 is produced in large quantities by the
|
feto-placental unit
|
|
|
Two sex hormones that play a role in the control of the menstrual cycle
|
estradiol and progesterone
|
|
|
Progesterone becomes critical during which phase of the menstrual cycle?
|
Luteal
|
|
|
Tests for ovulation check for the presence of
|
progesterone
|
|
|
FSH stimulates
|
follicles to grow
|
|
|
LH stimulates
|
ovulation
|
|
|
Corpus luteum secretes both
|
estradiol and progesterone
|
|
|
Functions of progesterone
|
1) Prepares uterus for implantation
2) Decreases maternal immune response 3) Decreases contractility of uterine smooth muscle 4) Helps maintain the pregnancy |
|
|
3 Neurosteroids
|
1) Progesterone
2) Pregnenolone 3) Dehydroepiandrosterone |
|
|
Starting material for all steroid hormones
|
Pregnenolone
|
|
|
Progesterone is a precursor for
|
aldosterone
|
|
|
Menstrual cycle is regulated by
|
LH and FSH (pituitary)
estrogen and progesterone (ovaries) |
|
|
Before 6 weeks gestation, what is the main source progesterone? After 12 weeks?
|
Corpus luteum.
Placenta |
|
|
Progesterone receptor antagonist
|
RU486
|
|
|
Enzyme which converts testosterone to DHT
|
5-alpha-reductase
|
|
|
Function of testosterone
|
1) sexual differentiation
2) spermatogenesis 3) development of secondary sexual organs, structures, and characteristics 4) anabolic metabolism and gene regulation 5) male-pattern behavior |
|
|
In bones, estradiol accelerates maturation of
|
cartilage into bone
|
|
|
GnRH stimulates production of
|
LH
|
|
|
LH stimulates testosterone production from
|
interstitial cells of the testes (Leydig cells)
|
|
|
Maturation of spermatozoa requires both
|
LH and FSH
|
|
|
FSH stimulates testicular growth and an androgen binding protein necessary for
|
sustaining the maturing sperm cell
|
|
|
Two possible pathways to testosterone
|
1) delta-4 (major): hydroxylation of progesterone -> side chain cleaved to form androstenedione
2) delta-5: pregnenolone->DHEA->androstenedione |
|
|
Immediate precursor of testosterone
|
androstenedione
|
|
|
Infants with 5-alpha-reductase disorder appear
|
female, lack of secondary sex characteristics. At puberty, females will virulize because of increase in testosterone levels
|
|
|
Proteins vs Steriods
|
see 40-136
|
|
|
Tubular organ lining epithelium and compact organs of the digestive system are derived from what germ layer?
|
Endoderm
|
|
|
The wall of the alimentary canal consists of four layers
|
mucosa, submucosa, muscularis, and adventitia
|
|
|
Alimentary Canal Mucosa: 2 types of epithelium
|
1) stratified squamous non-keratinized (protective)
2) simple columnar - secretoary/absorptive |
|
|
Alimentary Canal Mucosa: glands in surface epithelium
|
1) exocrine - goblet cells
2) endocrine - DNES (APUD) cells |
|
|
Alimentary Canal Mucosa: Characteristics of Lamina Propria
|
loose connective tissue with numerous reticular fibers. Part of immune system. GALT. Richly vascularized; fenestrated blood capillaries and lymphatic capillaries
|
|
|
Alimentary Canal Mucosa: Characteristics of Muscularis Mucosae
|
"thin smooth muscle; hallmark of esophagus, stomach, small/large intestine"
|
|
|
Alimentary Canal Muscularis Mucosae: Two layers of smooth muscle in muscularis mucosae
|
inner circular and outer longitudinal
|
|
|
Alimentary Canal Muscularis Mucosae: Function of muscularis mucosae
|
causes localized movements of mucosa which aid in digestion and absorption
|
|
|
Alimentary Canal Submucosa: Characteristics
|
"loose to moderately dense, irregular fibroelastic connective tissue (mostly type 1 collagen); GALT; contains largest blood and lymphatic vessels;"
|
|
|
Alimentary Canal Submucosa: Submucosal simple branched tubular glands are only present in
|
esophagus and duodenum
|
|
|
Alimentary Canal Submucosa: Submucosal plexus of Meissner
|
Parasym plexus synapses occur; also sympath. Fibers but no synapse. Controls motility of mucosa and secretory glands
|
|
|
Alimentary Canal Muscularis Externa
|
lips to upper 1/4 of esophagus = skeletal muscle; lower fourth of esophagus to rectum = smooth muscle. Anal canal = smooth muscle of internal sphincter and skeletal muscle of external sphincter
|
|
|
Alimentary Canal Muscularis Externa has
|
2 circular layers; peristaltic activity; inner circular control lumen; outer longitudinal shortens tube locally
|
|
|
Alimentary Canal Muscularis Externa: Myenteric plexus of Auerbach
|
2nd component of enteric nervous system; located between circular and longitudinal; parasymph gang. Synapses. Also sympath but no synapse
|
|
|
Alimentary Canal Adventitia: Characteristics
|
thin connective tissue (primarily type I collagen); sometimes covered by mesothelium; intraperitoneal = covered in serosa; retroperitoneal = behind serosa
|
|
|
The enteric nervous system is a self-contained nervous system composes of
|
numerous repeating ganglia known as the myenteric and submucosal plexuses
|
|
|
"The ENS, innervating the alimentary canal, is modulated by"
|
parasym and sym NS
|
|
|
"True or False. If the sym and parasym connection to the entire gut are severed, the alimentary canal can perform all of its functions without any major problems"
|
TRUE
|
|
|
Pacemakers which establish the rthythm of bowel contractions through their influence on electrical slow-wave activity are known as
|
interstitial cells of Cajal
|
|
|
The myenteric plexus of Auerbach is situated between
|
"muscle layers of the muscularis externa, contains neurons responsible for motility"
|
|
|
The smaller submucosal plexus of Meissner contains
|
"sensory cells that ""talk"" to the neurons of the myenteric plexus, and fibers that stimulate secretion from epithelial crypt cell into the gut lumen"
|
|
|
"True or False. The submucosal plexus contains fewer neurons and thinner interganglionic connectives (interneurons) than does the myenteric plexus, and has fewer neurons per ganglion."
|
TRUE
|
|
|
APUD cells in the gut secrete serotonin which acts to
|
"excite the mucosal afferent nerves, initiating the peristaltic reflex"
|
|
|
Neurotransmitters which cause inner circular layer of muscularis externa to contract
|
ACh and substance P
|
|
|
Neurotransmitters which cause inner circular layer of muscularis externa to dilate
|
vasoactive intestinal peptide (VIP) and nitric oxide
|
|
|
Increases peristalsis activity
|
parasympathetics
|
|
|
decreases peristasis activity
|
sympathetic
|
|
|
parasympathetic input comes from the … except in the colon and rectum where it comes from the …
|
"Vegas nerve, sacral outflow"
|
|
|
"Generally, parasympathetics"
|
"stimulate peristalsis, inhibit sphincters, trigger secretion"
|
|
|
sympathetic input comes from
|
splanchnic nerves (vasomotor)
|
|
|
"generally, sympathetics"
|
"inhibit peristalsis, activate sphincters"
|
|
|
Lining mucosa of oral cavity
|
stratified squamous nonkeratinized
|
|
|
Masticatory mucosa of oral cavity
|
stratified squamous parakeratinized
|
|
|
Specialized mucosa of oral cavity
|
stratified squamous nonkeratinized
|
|
|
stratified squamous parakeratinized is different because
|
stratum granulosum is missing and stratum corneum cells don't die
|
|
|
Specialized mucosa is specialized to
|
perceive taste
|
|
|
Lips are divided into 3 regions
|
"skin, vermilion border, mucosa"
|
|
|
V shaped groove between the body and root of tongue is called the
|
sulcus terminalis
|
|
|
"Extrinsic muscles of the tongue … the tongue, while intrinsic muscles … the tongue"
|
protrude/retrude. Alter the shape of
|
|
|
The dorsum of the tongue is uneven because of the presence of the
|
lingual tonsils
|
|
|
These are located in the anterior 2/3 of the tongue only
|
lingual papillae
|
|
|
Characteristics of filliform papillae
|
"sharp filimentous, most numerous, parakeratinized epithelium, contain tactile receptors but no taste buds"
|
|
|
Characteristics of fungiform papillae
|
"many blood vessels in LP, ~5 taste buds on upper surface, stratified squamous nonkeratinized"
|
|
|
Characteristics of vallate papillae
|
form an inverted V-shaped row proximal to terminal sulcus; ducts from von Ebner's glands empty into crypt; secrete lingual lipase; 100-300 taste buds on wall of crypts but not on upper surface; stratified squamous nonkeratinized
|
|
|
Characteristics of taste buds
|
"small intraepithelial structures; nearly 10,000; average lifespan of 10 days; entirely within epithelium not in LP; on fungiform,vallate papilla , soft palate, pharynx, larynx"
|
|
|
4 types of gustatory receptor cells
|
type IV (basal): unipotential stem cells; type I-III: taste cells synpase with nerve fibers; microvilli; neuroepithelial; SVA
|
|
|
Salty is detected by
|
Na+ enters through ion channels
|
|
|
Sour (acidic) is detected by
|
H+ enters through ion channels
|
|
|
Sweet and umami are detected by
|
G-protein
|
|
|
bitter is detected by
|
"G-protein (gustducin, similar to transducin)"
|
|
|
Fungiform papilla taste cells innervated by
|
CN VII
|
|
|
Vallate papilla taste cells innervated by
|
CN IX
|
|
|
Throat and epiglottis taste cells innervated by
|
CN X
|
|
|
3 glands of the tongue
|
1) Anterior Lingual 2) Posterial Lingual 3) von Ebner's glands
|
|
|
Posterior lingual glands are
|
mucous glands located in the root of the tongue
|
|
|
von Ebner's glands are
|
underlying the crypts surrounding the vallate papillae. All serous acini. Ducts open into crypts. Secrete lingual lipase.
|
|
|
# of permenant teeth
|
"32 (16 top, 16 bottom)"
|
|
|
# of deciduous teeth
|
"20 (10 top, 10 bottom)"
|
|
|
Makeup of permenant teeth
|
"8 incisors, 4 canines, 8 premolars, 12 molars"
|
|
|
Makeup of deciduous teeth
|
"8 incisors, 4 canines, 8 molars"
|
|
|
Dentin is a special hard connective tissue derived from
|
mesenchyme originating from neural crest
|
|
|
Only part of tooth derived from ectoderm
|
Enamel
|
|
|
Enamel-covered part of tooth is known as the
|
anatomical crown
|
|
|
Three divisions of the tooth
|
"crown, neck, root"
|
|
|
All three hard tissues of the tooth differ from bone in that they are
|
avascular
|
|
|
"Enamel differs from dentin, cementum, and bone in that it"
|
lacks collagen as its main organic component
|
|
|
Soft tissue in the middle of the tooth consisting of loose connective tissue supplied with numerous small blood vessels and nerve fibers that enter the apical foramen of the root canal is known as the
|
dental pulp
|
|
|
Cells responsible for producing dentin are the
|
odontoblasts
|
|
|
ligament which supports the tooth
|
periodontal ligament
|
|
|
periodontal ligament is richly vascularized and contains afferents sensitive to
|
pressure
|
|
|
bony socket in which the tooth is suspended by the periodontal ligament
|
alveolus
|
|
|
gomphosis is the
|
"dentoalveolar joint, classified as a synarthrosis (immovable joint)"
|
|
|
gingiva (gums) has what kind of epithelium?
|
stratified squamous parakeratinized
|
|
|
shallow ring-like groove where the gingiva borders the tooth
|
gingival sulcus
|
|
|
Bulk of the tooth
|
dentin
|
|
|
Dentin has a chemical composition similar to
|
bone
|
|
|
Hardest material found in the body consisting almost entirely of large apatite crystals
|
enamel
|
|
|
Enamel is produced prior to tooth eruption by
|
"ameloblasts, ectoderm-derived"
|
|
|
Cementum resembles bone except that it is
|
avascular
|
|
|
The palate is composed of the
|
"hard palate, soft palate, and uvula"
|
|
|
Characteristics of hard palate
|
"core of bone, lingual side is lined with stratified squamous parakeratinized"
|
|
|
Characteristics of soft palate
|
Core of skeletal musclel stratified squamous nonkeratinized
|
|
|
Characteristics of uvula
|
most posterior extension of soft palate; stratified squamous nonkeratinized
|
|
|
Three salivary glands
|
"parotid (ectoderm), submandibular (endoderm), sublingual (endoderm)"
|
|
|
parotid glands secrete
|
"serous from branched acinar glands, 25% of total salivary volume, Stensen's duct"
|
|
|
submandibular glands produce
|
"serous and mucus, 70% of salivary volume, branched tubuloacinar glands, serous demilunes, striated ducts, epidermal growth factor, Wharton's duct"
|
|
|
Sublingual gland secretes
|
"serous and mucous (mostly), 5% of salivary volume, no intercalated ducts, ducts of Rivinus"
|
|
|
2 types of interlobular ducts
|
1) intercalated - simple cuboidal 2) striated - simple columnar
|
|
|
Mostly from submandibular glands; about 1000 mL per day; parasym = greatly increase secretions - more watery; sym = moderately increase secretions - more viscous
|
saliva
|
|
|
Parotid glands innervated by
|
CN IX
|
|
|
Submandibular and sublingual glands innervated by
|
CN VII
|
|
|
"Primary saliva is of the same osmolarity as blood, but under the influence of"
|
aldosterone; can become hypotonic as Na+ is reabsorbed while passing through the striated ducts
|
|
|
Functions of saliva
|
"1) moisten and lubricate oral cavity 2) begins carbohydrate digestion via salivary amylase 3) aids in taste by dissolving food material 4) antibacterial activity - IgA, lactoferrin, lysozyme"
|
|
|
Middle oropharynx and laryngopharynx lined with
|
stratified squamous epithelium
|
|
|
"From the lips to the first 1/3 of the esophagus, what type of muscle?"
|
skeletal
|
|
|
Lining epithelium of esophagus is
|
stratified squamous nonkeratinized
|
|
|
Intersperse within esophagus epithelium are antigen-presenting cells known as
|
Langerhans cells
|
|
|
Muscularis mucosae in the esophagus is unusual in that it
|
consists of only a single layer of longitudinally oriented smooth muscle fibers that become thicker in the vicinity of the stomach
|
|
|
Mucous glands in the submucosa of the esophagus are the
|
esophageal glands proper
|
|
|
"Mucous glands in the lamina propria of the esophagus, especially inferior end"
|
esophageal cardiac glands
|
|
|
Muscularis externa of the esophagus contains
|
"only skeletal muscle in the upper 1/3, only smooth muscle in the lower 1/3, mixture of both in the middle third"
|
|
|
Outer surface of esophagus is covered by
|
"adventitia, except for the short serous-covered segment in the abdominal cavity between the diaphragm and stomach"
|
|
|
The esophagus has 2 physiological sphincters
|
1) pharyngoesophageal and 2) gastroesophageal
|
|
|
Four regions of the stomach
|
1) cardiac 2) fundus 3) body 4) pyloric antrum and canal
|
|
|
Region of the stomach which is the transition from the esophagus to the stomach
|
Cardiac region
|
|
|
Region of the Stomach which is above a horizontal line drawn from the cardia to the greater curvature
|
Fundus region
|
|
|
Region of the stomach which is located between the curvatures
|
Body region
|
|
|
"Region of the stomach which is the holding chamber, ends as the pylorus, contains pyloric sphincter"
|
Pyloric antrum and canal region
|
|
|
esophagus connects with the stomach at the
|
cardiac orifice
|
|
|
Stomach connects with the intestine at the
|
pyloric orifice
|
|
|
rugae are
|
major longitudinal folds in the lining of the stomach
|
|
|
the stomach is lined with
|
secretory simple columnar surface-lining cells that produce a neutral mucus product (NOT goblet cells)
|
|
|
(Stomach) epithelial sheet gland cells are attached by
|
tight junctions
|
|
|
Three types of gastric glands
|
1) Cardiac 2) pyloric 3) fundic
|
|
|
True or False. Gastric glands are exocrine glands without a true duct
|
TRUE
|
|
|
The gastric pit acts as the duct for 2-3 glands and is a surface invagination lined with
|
surface mucus epithelial cells
|
|
|
Cardiac glands secrete
|
mucus
|
|
|
Pyloric glands secrete
|
"mucus, lysozyme, and hormones"
|
|
|
Fundic glands are in the
|
body and fundus of the stomach
|
|
|
Five cell types of the fundic glands
|
1) Pluripotential stem cells 2) Mucous neck cells 3) Parietal cells 4) Chief cells 5) DNES Cells
|
|
|
Fundic glands: Pluripotential stem cells
|
have bidirectional migration; stimulated by APUD hormone 'gastrin' and EGF
|
|
|
Fundic glands: Mucous neck cells
|
in the neck only
|
|
|
Fundic glands: Parietal cells
|
"in the neck and base of the fundic gland; very large acidophilic, many mitochondria; invaginations of apical PM w/ microvilli = intracellular secretory canaliculus (only forms with cell is active)"
|
|
|
H+ is produced in the parietal cells by the enzyme
|
carbonic anhydrase
|
|
|
"In the parietal cells, H+ is pumped into the lumen of the intracellular canaliculus by a "
|
"H+, K+ ATPase. Simultaneously, K+ within the canaliculus is transported into the cell in exchange for the H+ ions. Cl- is transported through Cl- channels"
|
|
|
HCO3-/Cl- exchangers are located in the
|
"basolateral cell membrane, while H+,K+-ATPase is on the apical cell membrane"
|
|
|
#1 stimulator of acid production
|
histamine
|
|
|
Gastrin and histamine from APUD cells
|
stimulate acid production and release
|
|
|
Histamine comes from
|
APUD and mast cells
|
|
|
Histamine-blocking drugs
|
Tagamet and Zantac
|
|
|
Acetylcholine from parasympathetic
|
stimulates acid production
|
|
|
Prostaglandins inhibit
|
acid production
|
|
|
NSAIDs suppress
|
prostaglandin synthesis
|
|
|
Some APUD cells produce somatostatin which
|
inhibits the APUD gastrin producing cells and parietal cells
|
|
|
Parietal cells secrete gastric intrinsic factor (glycoprotein) which
|
binds to Vit B12 (needed for erythropoiesis) and is required for absorption in small intestine
|
|
|
Found only in the base region in the lower third of the fundic gland
|
Chief cells
|
|
|
"Have extensive RER in basal cytoplasm, apical cytoplasm contains zymogen granules that store pepsinogen, secrete gastric lipase"
|
Chief cells
|
|
|
Chief cells stimulated by
|
"everything that stimulates parietal cells (gastrin, histamine, parasympathetics)"
|
|
|
DNES cells produce and secrete
|
"histamine, gastrin, serotonin, VIP, and somatostatin"
|
|
|
DNES cells concentrate secretory granules in the
|
basal cytoplasm
|
|
|
"Stimulates parietal cells (acid), chief cells (pepsinogen)"
|
Histamine
|
|
|
affects smooth muscle cells (contraction stimulates motility) and parietal cells (inhibit acid secretion)
|
Serotonin
|
|
|
"Causes smooth muscle relaxation, blood vessel dilation, stimulates secretion and absorption"
|
vasoactive intestinal peptide (VIP)
|
|
|
"Stimulates parietal cells (acid), chief cells (pepsinogen), smooth muscle cells (increases gastric motility)"
|
Gastrin
|
|
|
"Inhibits release of other GI hormones, gastric acid and pepsinogen secretion, pancreatic exocrine secretion, salivary amylase secretion, intestinal motility, and contraction of gallbladder"
|
somatostatin
|
|
|
Stimulates pancreatic intralobular ducts to secrete HCO3- and water
|
Secretin
|
|
|
"Stimulates muscularis of gallbladder to contract and release bile, pancreatic acinar cells to secrete enzyme, sphincter of Oddi to relax"
|
Cholecystokinin (CCK)
|
|
|
Stimulates insulin secretion from pancreatic beta cells
|
Gastric inhibitory peptide (GIP)
|
|
|
"Stimulates gastric and duodenal motility, regulates contraction that occur in 2 hours cycles after meals"
|
Motilin
|
|
|
Bacteria which lives in the thick mucus and is primarily responsible for ulceration of the stomach
|
Helicobacter pylori
|
|
|
Substances absorbed by the stomach
|
"Water, salts, sugar, alcohol, drugs"
|
|
|
Predominantly produced in the stomach and stimulates appetite
|
Ghrelin
|
|
|
3 phases in gastric digestion
|
1) Cephalic 2) Gastric 3) Intestinal
|
|
|
The rate at which the stomach releases its chyme into the duodenum is a function of
|
"acidity, caloric and fat content, and osmolarity of chyme"
|
|
|
Factors that facilitate emptying
|
"distention of stomach, gastrin"
|
|
|
Factors that inhibit emptying
|
"distention of duodenum; over-abundance of fat, proteins, carbohydrates; increased osmolarity and excessive acidity of chyme"
|
|
|
CCK counteracts
|
gastrin
|
|
|
Meal time in stomach
|
carbs < proteins < fats; fats stimulate CCK which causes long setting in stomach
|
|
|
Digestion of chyme is primarily done in the
|
duodenum
|
|
|
Absorption of nutrients is primarily done in the
|
jejunum and proximal ileum
|
|
|
Production of hormones is by
|
APUD cells in the proximal small intestine
|
|
|
Small intestine specializations which increase surface area
|
"1) plicae circularis (circular folds) 2) intestinal villi (surface absorptive cells and goblet cells) lacteals, capillary loops 3) Microvilli (striated border microvilli)"
|
|
|
Invaginations of the epithelium into the lamina propria between the villi form intestinal glands known as
|
crypts of Lieberkuhn
|
|
|
Epithelium of the small intestine has 4 types of cells
|
1) Surface absorptive cells 2) Goblet cells 3) APUD cells 4) M cells
|
|
|
Apical surface of these cells presents with striated border microvilli
|
"Surface absorptive cells (tight junctions, absorpt much of the nutrients and fatty acids)"
|
|
|
"Unicellular glands, manufacture mucinogen"
|
Goblet cells
|
|
|
Produce paracrine and endocrine hormones
|
APUD cells
|
|
|
"These cells sample, phagocytose, and transport antigens present in the intestinal lumen"
|
M cells
|
|
|
Characteristics of LP of small intestine
|
"Part of GALT, numerous fenestrated capillaries, lacteals, some smooth muscle, intestinal crypts of Lieberkuhn(stem cells are here)"
|
|
|
Stem cells of the crypts of Lieberkuhn give rise to
|
"surface absorptive cells, goblet cells, APID cells, Paneth cells"
|
|
|
"Contain large acidophilic secretory granules, manufacture lysozyme, TNF-alpha, defensins, trypsin, and phospholipase A2"
|
Paneth cells
|
|
|
"These glands secrete a mucous, alkaline fluid in response to parasymp stimulation to help neutralize the acidic chyme that enters the duodenum"
|
Brunner's glands
|
|
|
The lamina propria and submucosa of the ileum house permanent clusters of lymphoid nodules known as
|
Peyer's patches
|
|
|
Monosaccharides and amino acids enter the absorptive cell through
|
microvillus membrane by transport proteins as a result of secondary active transport
|
|
|
Sugars and amino acids are released across the basal PM by
|
"facilitated diffusion, shuttled to the liver"
|
|
|
Long chain fatty acids and monoglycerides accumulate collect in the
|
"SER where they are reesterified to triglycerides, transferred to Golgi, form chylomicrons"
|
|
|
Lipid rich substance entering the lacteal is known as
|
Chyle
|
|
|
Permanent clusters of lymphoid nodules in the ileum are known as
|
Peyer's patches
|
|
|
Surface epithelium covering the lymphoid nodules are call
|
follicle-associated epithelium
|
|
|
Two additional cells found within the FAE
|
"Intraepithelial lymphocytes (most are helper T cells), and M cells"
|
|
|
Characteristics of large intestine
|
"plicae semilunares, crypts of Lieberkuhn, taeniae coli, haustra coli, no Paneth cells, no villi"
|
|
|
Apical membrane in large intestine have
|
Na+ and K+ channels responsible for Na+ absorption and K+ secretion.
|
|
|
Synthesis of Na+ channels is unduced by
|
aldosterone
|
|
|
"In diarrhea, high flow rate of intestinal fluid causes increases"
|
colonic K+ secretion (hypokalemia)
|
|
|
Colon also secretes
|
mucus and bicarbonate
|
|
|
"True or False. Epithelium of the anal mucosa is simple cuboid/columnar from the rectum to the pectinate line, first stratified columnar then stratified squamous nonkeratinized from the pectinate line to the external alan orifice, and stratified squamous keratinized (epidermis) at the anus"
|
TRUE
|
|
|
True or False. Lymph drainage is different above and below the pectinate line
|
TRUE
|
|
|
The exocrine pancreas is derived from
|
endoderm
|
|
|
Pancreatic acinar cells secrete
|
"trypsinogen (and trypsin inhibitor), chymotrypsinogen, procarboxypeptidase, proelastase, pancreatic amylase and lipase, deoxyribonuclease, ribonuclease"
|
|
|
Enteropeptidase converts
|
trypsinogen to trypsin
|
|
|
A distinguishing characteristic of the pancreas is the presence of cells in the beginning of the duct system called
|
centroacinar cells
|
|
|
These type of intalobular ducts are not found in the pancreas
|
striated ducts
|
|
|
common duct between pancreas and liver
|
ampulla of Vater
|
|
|
The intercalated ducts manufacture
|
"serous, bicarbonate-rich alkaline fluid"
|
|
|
Daily production of pancreatic juices is about
|
1200 mL
|
|
|
Secretin promotes
|
water and ion transport by stimulating intercalated duct cells
|
|
|
CCK stimulates acinar cells to synthesize and release
|
digestive enzymes
|
|
|
Major effects of CCK
|
"Stimulates secretion of pancreatic juice rich in digestive enzymes, causes ejection of bile from the gallbladder and opening of sphincter of Oddi, induces satiety"
|
|
|
Minor effects of CCK
|
"Inhibits gastric emptying, promotes normal growth and maintenance of pancreas, and enhances effects of secretin"
|
|
|
Liver is divided into 2 principle lobes by the falciform ligament
|
right and left
|
|
|
2 other lobes of the liver
|
quadrate and caudate lobes
|
|
|
Primary stromal fibers of the liver are
|
reticular fibers
|
|
|
Structures that enter the porta hepatis are
|
1) Hepatic portal vein 2) Hepatic artery proper 3) Autonomic nerves
|
|
|
Structures that exit the porta hepatis are
|
1) Left and right hepatic ducts 2) efferent lymphatics
|
|
|
Branch of the portal vein is called the
|
terminal portal venule
|
|
|
Branch of the hepatic artery is called the
|
terminal hepatic arteriole
|
|
|
Branch of the biliary duct system is called the
|
bile ductule (usually lined with cuboidal epithelium)
|
|
|
Lymphatics are generally one or two small
|
lymphatic capillaries
|
|
|
Portal areas refer to sites within the septa that contain the five structures
|
1) terminal portal venule 2) terminal hepatic arteriole 3) bile ductule 4) lymphatic capillaries 5) nerve branches
|
|
|
A continuous wall of hepatocytes which surrounds entire interlobular septum is called the
|
limiting plate
|
|
|
The space between the connective tissue and the limiting plate is known as
|
periportal tissue space of Mall
|
|
|
Basic functional unit of the parenchyma of the liver is the
|
classic lobule
|
|
|
"Within lobules, hepatocytes are arranged in"
|
rows or radiating plates (chords) - 1 or 2 cells thick
|
|
|
Hepatocytes radiate out from a blood vessel in the center of the lobule called the
|
central vein
|
|
|
"Running between the hepatocytes cords, the liver has a special type of blood vessel that called the"
|
sinusoid
|
|
|
The primary vascular bed of the liver portal system is in the intestine (fenestrated capillaries). The secondary vascular bed consists of
|
the sinusoids in the liver
|
|
|
The arrangement in the liver is known as the
|
hepatic portal system
|
|
|
The blood in the sinusoids carries all of the absorbed materials from the intestines except for
|
the bulk of the lipid
|
|
|
Every hepatocyte makes at least 1 contact with
|
a sinusoid
|
|
|
Triad consists of
|
"hepatic arteriole, bile duct, portal venule"
|
|
|
True or False. Blood always flows from the periphery of the lobules (the portal areas) ->sinusoids -> central vein
|
TRUE
|
|
|
The liver is the only place in the body that has blood vessels lined by endothelial cells and
|
macrophages
|
|
|
Endothelial cells of sinusoids are highly fenestrated and have
|
very large pores without diaphragms
|
|
|
"Fixed macrophages, making up the lining of the sinusoidal wall, are called"
|
Kupffer cells
|
|
|
Space between the sinusoids and plates of hepatocytes is called the
|
perisinusoidal space of Disse
|
|
|
Contents of the perisinusoidal space (5)
|
1) tissue fluid 2) nerve fibers 3) fibroblasts 4) reticular fibers 5) hepatic stellate cells
|
|
|
True or False. Tissue fluid and plasma have the same composition
|
TRUE
|
|
|
True or False. Tissue fluid gives rise to all the lymph produced in the liver
|
TRUE
|
|
|
Hepatic stellate cells secrete
|
"type I collagen, laminin, proteoglycans, and growth factors"
|
|
|
Cirrhosis is
|
increased deposition of collagen and ECM components
|
|
|
"As the fibrotic process advances in the liver, hepatic stellate cells change into … constricting the lumen of the sinusoids and increasing vascular resistance"
|
myofibroblasts
|
|
|
An increase in resistance to flow of portal venous blood in the hepatic sinusoids leads to
|
portal hypertension in cirrhosis
|
|
|
"Under normal circumstances, hepatic stellate cells"
|
store fat-soluble vitamin A and produce collagen fibers and ECM components deposited in the space of Disse and around the central vein of the lobule
|
|
|
Lymphatic drainage pathway
|
"Plasma exits sinusoids into space of Disse (tissue fluid). Then leaks between hepatocytes into space of Mall, then seeps into connective tissue of portal area. Next, it enters lymphatic capillaries where it is now called ""lymph"""
|
|
|
Exocrine product of liver is
|
"bile, produced by hepatocyte"
|
|
|
Bile is released into the
|
bile canaliculus
|
|
|
True or False. Flow of bile is ALWAYS from the center of the lobules toward the peripheral portal areas
|
TRUE
|
|
|
"Very close to the limiting plate is the beginnings of the first true duct, which is the"
|
preductile canal of Hering
|
|
|
The preductile canal of Hering goes through the limiting plate and join with the
|
bile ductule in the portal area
|
|
|
Characteristics of Hepatocytes
|
"1) single nucleus (80%) or binucleates (20%) 2) 75% are polyploid (endoreduplication) 3) many mitochondria, Golgi, RER, SER, lysosomes, peroxisomes 4) poorly staining cytoplasm"
|
|
|
Hepatocytes have a life span of about
|
150 days
|
|
|
Surface adjacent to other hepatocytes (15% of cell surface) has
|
many desmosomes (hold cells together) and gap junctions (communicate with neighbors)
|
|
|
Cell surface bordering intercellular canaliculi (15% of cell surface) (secretory surface) contains
|
tight junction (close off the canaliculi)
|
|
|
Surface bordering the space of Disse (70% of cell surface) (secretory and absorptive surface) has
|
microvilli
|
|
|
Functions of hepatocytes
|
1) Secrete bile 2) Protein catabolism 3) Carbohydrate metabolism 4) Lipid metabolism 5) Detoxify drugs and alcohol
|
|
|
Two important components of bile
|
bile salts and bilirubin
|
|
|
Liver can convert what to produce glucose
|
"glycogen, amino acids, lactic acid, fructose, galactose"
|
|
|
Shape of liver acinus is
|
ovoid to diamond; two central veins at each end with two portal areas at the other two ends
|
|
|
The backbone of the acinus supply is the
|
arterial blood in branches of hepatic arters
|
|
|
Zone 1 (perilobular region) has
|
"highest nuterient and oxygen, most metabolically active, oxidative metabolism here. Active synthesis of glycogen and plasma proteins, most resistant to insult, first to regenerate"
|
|
|
Zone 2
|
intermediate region
|
|
|
Zone 3
|
"Cells closest to central vein, lowest in oxygen and nutrients, highest in metabolic wastes. Glycolysis happening here. Principle site of alcohol and drug detox. More vulnerable to damage"
|
|
|
Lipid deposits in zone 3 hepatocytes may indicate
|
consumption of hepatotoxic substances
|
|
|
Centrilobular necrosis of the liver is most likely to occur as a result of
|
"malnutrition, drug and chemical toxicity, or ischemic injury"
|
|
|
Function of gallbladder
|
store and concentrate bile
|
|
|
gallbladder epithelium
|
"simple columnar, interdigitation. Mucosa reabsorbs water and ions from bile"
|
|
|
"In the gallbladder, epithelial clefts or diverticula called ... extend into the wall"
|
Rokitansky-aschoff crypts
|
|
|
Bile duct system
|
right + left hepatic ducts -> common hepatic duct + cystic duct -> common bile duct + pancreatic duct -> ampulla of Vater --> sphincter of Oddi
|
|
|
Regulation of bile secretion is through
|
parasympathetics and CCK
|
|
|
CCK causes ejection of bile from gallbladder and relaxation of
|
sphincter of Oddi
|
|
|
Five major processes of the digestive system
|
Ingestion, Motility, Digestion, Absorption, Elimination
|
|
|
In the enteric nervous system, ACh causes (3)
|
1) Contraction of smooth muscle in wall of digestive tract
2) Relaxation of sphincters 3)Increased salivary, gastric acid and pancreatic secretions |
|
|
In the enteric nervous system, Norepi causes (3)
|
1) Relaxation of smooth muscle in wall of digestive tract
2) Contraction of sphincters 3) Increased mucus secretion in saliva |
|
|
In the enteric nervous system, Vasoactive Intestinal Polypeptide causes (2)
|
1) Relaxation of smooth muscle in wall of digestive tract
2) Increased small intestinal and pancreatic secretions |
|
|
In the enteric nervous system, Gastrin-Releasing Polypeptide causes (1)
|
1) Increased gastrin secretion
|
|
|
In the enteric nervous system, Enkephalins cause (2)
|
1) Contraction of smooth muscle in wall of digestive tract
2) Decreased intestinal secretion |
|
|
In the enteric nervous system, Neuropeptide Y causes
|
1) Relaxation of smooth muscle in wall of digestive tract
2) Decreased intestinal secretion |
|
|
In the enteric nervous system, Substance P causes (2)
|
1) Contraction of smooth muscle
2) Increased salivary secretion |
|
|
Vegas provide parasympathetics to which portion of the digestive tract?
|
Upper (up to left colic flexure)
|
|
|
Sacral outflow provides parasympathetics to which portion of the digestive tract?
|
Lower (descending colon, sigmoid colon, rectum)
|
|
|
Submucosal and myenteric plexuses are innervated by
|
parasym and sym
|
|
|
Parasympathetic neurotransmitters
|
ACh, Substance P, Vasoactive intestinal peptide
|
|
|
Sympathetic neurotransmitters
|
Norepinephrine
|
|
|
Four sympathetic ganglia serve the digestive tract
|
Celiac, superior mesenteric, inferior mesenteric and hypogastric
|
|
|
During mastication, sensory information is relayed via
|
CN V
|
|
|
Muscles of mastication are innervated by
|
CN V
|
|
|
Three phases of deglutition (swallowing)
|
Oral (voluntary), Pharyngeal (involuntary), Esophageal (involuntary)
|
|
|
Cranial nerves involved in swallowing 1) sensation 2) motor
|
1) V, IX, X
2) V, IX, X, XII |
|
|
Upper esophageal sphincter prevents
|
reflux of bolus back into pharynx
|
|
|
During swallowing, 1) soft palate and 2) epiglottis are coverings for
|
1) nasopharynx
2) larynx |
|
|
Slow waves originate into the
|
interstitial cells of Cajal
|
|
|
Slow waves are
|
oscillating depolarization and repolarization of the membrane potential of the smooth muscle cells.
|
|
|
Phasic contractions happen when
|
action potential threshold is met (strong)
|
|
|
Tonic contractions happen when
|
action potential threshold is not met
|
|
|
Interstitial cells of Cajal are electrically connected to smooth muscle via
|
gap junctions
|
|
|
Upswing of the slow wave is due to
|
inward Ca++ current due to gating of voltage-gated Ca++ channels
|
|
|
Plateau of the slow wave is due to
|
inward Ca++ current due to gating of L-type Ca++ channels
|
|
|
Downswing of the slow wave is due to
|
outward K+ current due to gating of voltage-gated K+ channels
|
|
|
Frequency of slow waves in the stomach and colon
|
3 per minute
|
|
|
Frequency of slow waves in the duodenum
|
12 per minute
|
|
|
True or False. Frequency of slow waves is not affected by neural or hormonal input.
|
True
|
|
|
Periodic contractions followed by relaxation (organized by slow waves); propel and mix contents
|
phasic contractions
|
|
|
Maintain a constant level of contraction of tone ("latch state"); found in sphincters
|
tonic contractions
|
|
|
Primary peristaltic contractions in the esophagus are mediated by
|
the swallowing reflex
|
|
|
If swallows are taken in rapid succession, esophageal motility is inhibited. This is called
|
deglutitive inhibition
|
|
|
Opening of lower esophageal sphincter is caused by
|
primary peristaltic contractions and mediated by the vegas nerve which relaxes it
|
|
|
Secondary peristaltic contractions are
|
a second series of contractions (if needed) to clear remaining contents
|
|
|
Secondary peristaltic contractions begin at
|
point of distention
|
|
|
What happens in receptive relaxation?
|
1) Orad region of the stomach relaxes (mediated by vegas)
2) Decreases pressure and increases volume of orad making it easier for bolus to enter |
|
|
Mixing starts in caudad region of stomach and moves toward
|
antrum, getting stronger along the way
|
|
|
Full gastric emptying takes approximately
|
3 hours
|
|
|
True or False. Liquids empty from the stomach quicker than solids.
|
True
|
|
|
True or False. Isotonic chyme empties from the stomach quicker than hypo or hypertonic chyme.
|
True
|
|
|
True or False. Carbohydrates empty from the stomach the fastest while lipids empty the slowest.
|
True
|
|
|
Motilin mediates
|
migrating myoelectric complex, occuring an 90 minute intervals
|
|
|
Ductal cells modify saliva by
|
secreting K+ and HCO3-, and absorbing Na+ and Cl-
|
|
|
Ductal cells are impermeable to
|
water
|
|
|
Osmolarity of saliva
|
100 mOsm
|
|
|
4 organic components of saliva
|
1) salivary amylase
2) lingual lipase 3) mucin glycoprotein 4) antibodies (IgA) and lysozyme |
|
|
Alkaline tide is caused by
|
gastric venous blood
|
|
|
Proton pumps transfer H+ into the lumen and ... into the cell
|
K+
|
|
|
Gastric secretions include (4)
|
1) Pepsinogen
2) Intrinsic Factor 3) Gastric Lipase 4) Mucus |
|
|
Pepsinogen is secreted by
|
Chief cells
|
|
|
Function of pepsin
|
digest protein
|
|
|
Intrinsic factor is secreted by
|
parietal cells
|
|
|
Intrinsic factor is necessary for
|
proper vitamin B12 absorption in the ileum
|
|
|
Lack of intrinsic factor leads to
|
pernicious anemia
|
|
|
Parietal cells are located in the body of the stomach and secrete
|
HCl and Intrinsic factor
|
|
|
Chief cells are located in the body of the stomach and secrete
|
Pepsinogen
|
|
|
G-cells are located in the antrum of the stomach and secrete
|
Gastrin
|
|
|
Mucous cells are located in the antrum of the stomach and secrete
|
Mucus and pepsinogen
|
|
|
Protein digestion in the stomach is done by
|
pepsin
|
|
|
Protein digestion in the small intestine is done by
|
trypsin, chymotrypsin, elastase, carboxypeptidase A, carboxypeptidase B and peptidases in the brush border
|
|
|
Triglycerides are broken down by
|
lingual, gastric, and pancreatic lipases
|
|
|
cholesterol ester is broken down by
|
cholesterol ester hydrolase
|
|
|
Phospholipid is broken down by
|
phospholipase A2 into lysolecithin and fatty acid
|
|
|
Glucose is absorbed by
|
Na+-glucose cotransport via SGLT-1 cotransporter via secondary active transport on apical membrane. Then facilitated diffusion via GLUT-2 transporter on basolateral membrane
|
|
|
Galactose is absorbed by
|
Na+-galactose cotransport via SGLT-1 cotransporter via secondary active transport on apical membrane. Then facilitated diffusion via GLUT-2 transporter on basolateral membrane
|
|
|
Fructose is absorbed by
|
Facilitated diffusion via GLUT-5 cotransporter on apical membrane. Facilitated diffusion via GLUT-2 transporter on basolateral membrane
|
|
|
There are 4 separate transporters/cotransporters for amino acids
|
1 for basic, 1 for acidic, 1 for neutral, and 1 for imino
|
|
|
Amino acids first get into cell by
|
Na+/amino acid cotransporter, then out by facilitated diffusion through amino acid transporter
|
|
|
Most protein absorption is in the form of
|
di and tripeptides
|
|
|
Apical membrane peptide transport is through
|
H+/peptide cotransporter (one for di and one for tri)
|
|
|
Basal membrane peptide transport is
|
same as amino acids because the peptides are broken down once in the cell
|
|
|
Absorbed lipids are (4)
|
1) Cholesterol
2) Monoglycerides 3) Lysolecithin 4) Free Fatty Acids |
|
|
All products of lipid digestion are contained within
|
micelles
|
|
|
Once mycelle-released lipids diffuse across the apical membrane, they
|
are re-esterified with free fatty acids to form original ingested lipids
|
|
|
Lipids are packaged with apoproteins and together are known as
|
chylomicrons
|
|
|
Chylomicrons are packaged in secretory vesicles in the
|
Golgi
|
|
|
Chylomicrons are exocytosed into
|
lacteals
|
|
|
External respiration is 1)... where as internal respiration is 2)...
|
1) Exchange of O2 in the inspired air for CO2 in the blood
2) Exchange of CO2 for O2 in the vicinity of the cells |
|
|
Upper respiratory system includes
|
nose and pharynx
|
|
|
Lower respiratory system includes
|
larynx, trachea, bronchi, and lungs
|
|
|
Functionally the respiratory system consists of 2 parts
|
1) conducting portion and 2) respiratory portion
|
|
|
Main sites of gas exchange
|
alveoli
|
|
|
Volume of the conducting portion
|
150mL
|
|
|
Volume of the respiratory portion
|
5-6L
|
|
|
Starting with the trachea, the air may pass through as many as ... generations of branchings
|
23
|
|
|
The first 16 generations of airways are in the ... zone
|
conducting
|
|
|
Even though Poiseuille's law suggest that the smallest airways have the highest resistance to airflow, the do not because of
|
their parallel arrangement
|
|
|
Four elements which aid in respiration
|
1) rib cage
2) diaphragm 3) intercostal muscles 4) elastic connective tissue of the lung |
|
|
Normal quiet inhalation involves contraction of what muscles of inhalation?
|
diaphragm and external intercostals
|
|
|
During normal quiet breathing, exhalation results from
|
elastic recoil
|
|
|
During normal quiet breathing, exhalation results from
|
elastic recoil
|
|
|
Normal quiet breathing is called
|
eupnea
|
|
|
Average healthy adult tidal volume
|
500mL
|
|
|
Characteristic epithelium of the conducting portion is
|
pseudostratified ciliated columnar
|
|
|
The respiratory epithelium is composed of 5 cell types
|
1) Goblet cells - mucus
2) Ciliated solumnar cells-move mucus toward oropharynx 3) Basal cells - stem cells 4) Brush cells 5) DNES cells - regulation |
|
|
Nasal cavity is made up of 4 parts
|
1) External nares
2) Vestibule 3) Nasal cavity proper 4) Olfactory Mucosa |
|
|
Divided by nasal septum; mostly lined by typical respiratory epithelium; communicates with pharynx through choanae; vascular lamina propria; countercurrent head exchange system
|
Nasal cavity proper
|
|
|
Swell bodies are
|
a large venous plexus which engorge on one side every half hour to allow mucosal rehydration
|
|
|
Conchae cause
|
airflow turbulence
|
|
|
Where the lamina propria blends with the periosteum of the underlying bone in the nasal cavity proper is called
|
mucoperiosteum
|
|
|
Olfactory epithelium is tall
|
pseudostratified epithelium
|
|
|
Three cell types if olfactory mucosa
|
1) Olfactory receptors - middle 1/3
2) Supporting cells - upper 1/3 3) Basal cells - stem cells - lower 1/3 |
|
|
Olfactory cilia and receptors for odors and are linked to
|
G-proteins: cAMP->open Na+ channels->nerve impulse
|
|
|
Are olfactory receptors are myelinated?
|
no
|
|
|
Produces thin, watery fluid that is carried to the surface of the olfactory epithelium which contains lysozyme, IgA and odorant-binding protein
|
olfactory glands of Bowman
|
|
|
Perinasal sinuses are lined with
|
respiratory epithelium
|
|
|
Boundaries of the nasopharynx
|
Choanae and soft palate
|
|
|
Nasopharynx is lined with
|
respiratory epithelium
|
|
|
oro and laryngeopharynx are lined by
|
stratified squamous nonkeratinized epithelium
|
|
|
Hyaline cartilages of the larynx
|
Thyroid, cricoid, and lower part of arytenoids
|
|
|
Elastic cartilages of the larynx
|
Epiglottis, corniculate, and tips of arytenoids
|
|
|
True and false vocal chords are covered by
|
stratified squamous nonkeratinized epithelium
|
|
|
False vocal chords are positioned 1) ... while the true vocal chords are positioned 2) ...
|
1) superiorly
2) inferiorly |
|
|
Between the true and false vocal chords is a recessed region called the
|
vestibule
|
|
|
True or False. There are no glands in the lamina propria of the true vocal chords.
|
True
|
|
|
Inferior to the true vocal chords the lining epithelium changes to
|
respiratory epithelium
|
|
|
Boundaries of the trachea
|
from cricoid cartilage until bifurcation
|
|
|
Tracheal walls are supported by
|
10-12 C-rings
|
|
|
Dense fibroelastic connective tissue between adjacent C-rings permits
|
elongation of the trachea during inhalation
|
|
|
Contraction of the trachealis (smooth muscle) decreases the diameter of the trachea, resulting in faster airflow, which
|
assists in the dislodging of foreign matter from the larynx during coughing
|
|
|
The trachea consists of three layers
|
1) Mucosa - respiratory epithelium - thick basement membrane
2) Submucosa - dense irregular fibroelastic w/ seromucous glands 3) Adventitia - C-rings |
|
|
The bronchial tree begins at the
|
bifurcation of the trachea, as right and left primary bronchi, which arborize
|
|
|
The airways outside of the lungs are
|
the primary bronchi, extrapulmonary bronchi
|
|
|
The airways inside of the lungs are
|
the intrapulmonary bronchi, conducting bronchioles, terminal bronchioles, and respiratory bronchioles.
|
|
|
With successive branching, there is a decrease in the diameter of the
|
lumen, but an increase in total surface area
|
|
|
Decreased airway diameter means decreases cartilage but
|
increased smooth muscle and elastic fibers
|
|
|
Decreased airway diameter means decreases
|
cilia (height and number) and glands
|
|
|
Have mixed glands, O-rings of cartilage, accompanied by pulmonary arteries, veins, and lymph vessels. Pierces the hilus of the lung.
|
Primary (Main) bronchi
|
|
|
The left main bronchus bifurcates but the right main bronchus
|
trifurcates
|
|
|
secondary bronchi is the airway to the
|
lobes (AKA lobar bronchi)
|
|
|
As secondary bronchi enter the lobes of the lung, they subdivide into smaller branches called
|
tertiary bronchi
|
|
|
Each tertiary bronchus arborizes but leads to a discrete section of lung tissue known as a
|
bronchopulmonary segment
|
|
|
How many bronchopulmonary segments does each lung have?
|
10
|
|
|
Conducting bronchioles supply air to a
|
pulmonary lobule. Lack cartilage and glands.
|
|
|
Epithelium of conducting bronchiole is
|
simple solumnar ciliated with goblet cells
|
|
|
Has prominent thick layers of helically oriented smooth muscle
|
Conducting bronchioles
|
|
|
Sympathetic stimulation of bronchiolar smooth muscle causes
|
relaxation and increases the diameter of the airways (beta-2 adrenergic receptors)
|
|
|
Parasympathetic stimulation of bronchiolar smooth muscle causes
|
constriction and decreases the diameter of the airways. Also caused by histamine, leukotrienes, and prostaglandins
|
|
|
Each conducting bronchiole subdivides to form smaller
|
terminal bronchioles, which constitute the end of the conducting portion of the respiratory system.
|
|
|
Terminal bronchioles have epithelium of
|
ciliated simple cuboidal and lacks goblet cells
|
|
|
Clara cells are
|
columnar with dome-shapes apices that have short, blunt microvilli. Secretory granules, anti-inflammatory.
|
|
|
Functions of clara cells
|
1) secrete glycoprotein
2) secrete surfactant-like product 3) secrete enzymes 4) release Cl- 5) Stem cells |
|
|
First part of the respiratory portion. Simple cuboidal, clara cells with some cilia (last place for cilia)
|
respiratory bronchioles
|
|
|
In the respiratory bronchioles, usually one side of the wall is interrupted by
|
alveoli. In the connective tissue of the opposite wall is a branch of the pulmonary artery
|
|
|
walls consist of nothing but alveoli
|
alveolar ducts
|
|
|
The respiratory passages from the trachea to the alveolar ducts contain about
|
25 orders of branching
|
|
|
Expanded outpouchings of numerous alveoli located at the distal ends of alveolar ducts. No smooth muscle in the walls.
|
Alveolar sacs
|
|
|
Alveoli are separated from each other by
|
interalveolar septa which contain one or more alveolar pores.
|
|
|
Alveoli are rimmed by
|
elastic fibers
|
|
|
Alveoli are lined by
|
highly attenuated simple squamous epithelium composed of type I and type II pneumocytes
|
|
|
about 95% of the alveolar surface is composed of simple squamous made up of
|
type I pneumocytes
|
|
|
These cells have tight junctions, thin cytoplasm, function in gas exchange, cannot divide
|
type I pneumocytes
|
|
|
Cuboidal in shape, microvilli, tight junction, can divide into both types of pneumocytes
|
type II pneumocytes
|
|
|
Secretory product of type II pneumocytes is
|
pulmonary surfactant = DPPC
|
|
|
Alveolar macrophages make this enzyme which breaks down elastic fibers
|
elastase
|
|
|
Alveolar macrophages of patients with pulmonary congestion and congestive heart failure contain phagocytosed, extravasated red blood cells, and are referred to as
|
heart failure cells
|
|
|
Interalveolar septum has 2 regions
|
1) Thick - continuous capillaries
2) Thin - form blood-air barriers |
|
|
Blood-air barriers consist of 3 payers
|
1) surfactant and type I pneumocytes
2) Fubed basal laminae of type I pneumocytes and capillary endothelial cells 3) Endothelium of continuous capillaries |
|
|
True or False. In the lung lobules, pulmonary veins are separated from the arteries
|
True
|
|
|
Pulmonary vascular supply
|
1) Pulmonary artery
2) Pulmonary veins 3) Bronchiole arteries and veins |
|
|
Pulmonary nerve supply
|
autonomic nerve fibers to smooth muscle of bronchi and bronchioles. PNS causes contraction. SNS causes relaxation.
|
|
|
External respiration is
|
gas exchange between the lungs and the blood
|
|
|
Internal respiration is
|
gas exchange between the blood and body cells
|
|
|
Zones 1-16 are
|
conducting zones
|
|
|
Zone 17-23 are
|
respiratory zones
|
|
|
Relax smooth muscle, dilate brochioles via beta-2 adrenergic receptors
|
sympathetics
|
|
|
contract smooth muscle, constrict bronchioles vis muscarinic receptors
|
parasympathetics
|
|
|
beta-2 agonist
|
Albuterol
|
|
|
muscarinic antagonist/anchicholinergic
|
Ipratropium
|
|
|
beta-2 agonists and anticholinergics work to
|
treat asthma
|
|
|
Last place in the respiratory system where cilia is found
|
respiratory bronchioles
|
|
|
Last place in the respiratory system where smooth muscle is found
|
alveolar ducts
|
|
|
Last place in the respiratory system where cartilage is found
|
bronchi
|
|
|
Tidal volume (500mL) is the
|
volume of air inspired or expired during normal, quiet breathing
|
|
|
Inspiratory reserve volume (3.0-3.3L) is the
|
maximum amount of air that can be inspired at end of a normal inspiration
|
|
|
Expiratory reserve volume (1.0-1.2L) is the
|
maximum amount of air that can be expired at end of a normal expiration
|
|
|
Residual volume (~1.2L) is the
|
volume of air remaining in the lungs after a forced expiration
|
|
|
Total lung capacity (5.7L-6.2L) is the
|
volume of air in the lungs after a maximal inspiration
|
|
|
Vital capacity (4.5L-5.0L) is the
|
volume of air forcefully expired from lungs after a maximal inspiration
|
|
|
Functional residual capacity (2.2L-2.4L) is the
|
volume of air remaining in the lungs after a normal expiration
|
|
|
Inspiratory capacity (3.5L-3.8L) is the
|
volume of air that can be inspired after a normal expiration
|
|
|
FVC (5L) is the
|
volume of air forcefully expired after a maximal inspiraction
|
|
|
FEV1 (4L) is the
|
volume of air forcefully expired in the first second
|
|
|
FEV3 (4.75L) is the
|
volume of air forcefully expired in three seconds
|
|
|
FEV1/FVC * 100% =
|
80%
|
|
|
1 cm H2O ~=
|
0.74 mmHg
|
|
|
Compliance (dV/dP) is the
|
measure of how volume changes as a result of a pressure change
|
|
|
Normal compliance value of the lung is
|
0.13 liters / cm H2O
|
|
|
Compliance is directly/inversely proportional to elastance.
|
inversely
|
|
|
Increase in lung compliance happens in
|
emphysema. Loss of elastic fibers, Elastance decreases, lung volumes are increased
|
|
|
Decrease in lung compliance is seen in
|
Fibrosis. Stiffening of lung tissue, lung resists expansion, lung volumes are decreased.
Infant respiratory distress syndrome. Lack of pulmonary surfactant. Increased surface tension. Difficult to keep alveoli inflated. Atelectasis (collapse of alveoli) |
|
|
Changing radius two fold will change air flow
|
sixteen fold
|
|
|
Change in pressure for tidal volume is approximately
|
1-2 cm H2O
|
|
|
Pleural pressure is always less than
|
alveolar pressure. Aids in keeping alveoli inflated
|
|
|
Pleural pressure is produced by
|
tendency of lungs to recoil and chest wall to expand
|
|
|
Transpulmonary pressure is
|
pressure across the alveoli and airways (Ppl - Palv)
|
|
|
External respiration occurs between
|
alveoli and pulmonary capillaries
|
|
|
Anatomical dead space (conducting zone volume) is approximately
|
volume in mL is equal to weight in pounds
|
|
|
Alveolar dead space is due to
|
lack of blood supply or damage to alveoli
|
|
|
Physiological dead space is a combination of
|
anatomical and alveolar dead spaces
|
|
|
Partial pressure exerted by gaseous water molecules
|
47 mmHg
|
|
|
[Dissolved gas]=
|
(Pgas) X (solubility coefficient of gas)
|
|
|
Partial pressure of oxygen in dry air
|
160 mmHg
|
|
|
Partial pressure of CO2 in dry air
|
negligible
|
|
|
Partial pressure of H2O in conducting zone
|
47 mmHg
|
|
|
Partial pressure of O2 in conducting zone
|
150 mmHg
|
|
|
Partial pressure of CO2 in conducting zone
|
30 mmHg
|
|
|
Partial pressure of O2 in alveoli
|
104 mmHg
|
|
|
Partial pressure of CO2 in alveoli
|
40 mmHg
|
|
|
Partial pressure of O2 in arterial blood
|
95 mmHg or torr
|
|
|
Partial pressure of CO2 in arterial blood
|
40 mmHg or torr
|
|
|
Partial pressure of O2 in venous blood
|
40 mmHg or torr
|
|
|
Partial pressure of CO2 in venous blood
|
45 mmHg or torr
|
|
|
Partial pressure of O2 in interstitial space
|
40 mmHg or torr
|
|
|
Partial pressure of CO2 in interstitial space
|
45 mmHg or torr
|
|
|
Partial pressure of O2 in cells
|
20 mmHg or torr
|
|
|
Partial pressure of CO2 in cells
|
46 mmHg or torr
|
|
|
Diffusion of gas across a membrane is directly proportional to
|
diffusion coefficient, surface area, pressure gradient
|
|
|
Diffusion of gas across a membrane is inversely proportional to
|
membreane thickness
|
|
|
Body needs approximately ...mL of O2/min
|
250
|
|
|
Oxygen transferred in blood as
|
98% bound to hemoglobin, 2% dissolved in plasma
|
|
|
Percentage of hemoglobin saturation @ venous block PO2 (40 mmHg)
|
75%
|
|
|
Percentage of hemoglobin saturation @ arterial blood PO2
|
98%
|
|
|
Inc PCO2, dec pH, inc temp, inc 2,3-DPG cause a shift in the oxygen-hemoglobin dissociation curve to the
|
right.
|
|
|
Inc PCO2, dec pH, inc temp, inc 2,3-DPG cause a shift in the oxygen-hemoglobin dissociation curve to the
|
right.
|
|
|
As more oxygen is bound to Hb, more CO2 will be released from Hb which is known as the
|
Haldane effect (occurs at the lungs)
|
|
|
70-90% of CO2 is transported as
|
HCO3-
|
|
|
This contains a respiratory center that shapes breating. Bilateral network of meurons with pacemaker-like activity throughout it.
|
Medulla
|
|
|
Voluntary aspect of breathing
|
Cerebral cortex
|
|
|
Emotional aspect of breathing
|
Hypothalamus
|
|
|
Regulates duration of inspiration (shortens inspirations)
|
Pontine respiratory group / Pneumotaxic center
|
|
|
Central chemoreceptors are located in multiple areas of the
|
medulla and pons
|
|
|
Peripheral chemoreceptors (glomus cells) are located in walls of
|
carotid arteries (carotid body)
|
|
|
Strongest chemostimulus to breathing
|
CO2 / pH
|
|
|
Located throughout the airways and alveoli. Detect the amount of stretch in the lungs
|
Pulmonary stretch receptors
|
|
|
Located throughout limbs and the body
|
Proprioceptors and exteroreceptors
|
|
|
Kidneys are intraperitoneal or retroperitoneal
|
retroperitoneal (as is the entire urinary system)
|
|
|
Kidneys are at approximately what spinal level?
|
T12
|
|
|
Blood pressure is regulated by the kidneys through the
|
renin-angiotensin system
|
|
|
The kidneys also secrete 1) Erythropoietin and 2) Calcitriol which do what?
|
1) Stimulate red blood synthesis in the blood marrow
2) Regulates calcium homeostasis |
|
|
Waste products excreted in urine include
|
ammonia, urea, bilirubin, creatinine, uric acid, foreign substances such as drugs and environmental toxins
|
|
|
Renal sinus includes
|
Major and minor calyx, and renal pelvis
|
|
|
Kidneys are encapsulated by a
|
thin dense irregular connective tissue capsule
|
|
|
Seven structures of the kidney
|
1) Renal sinus
2) Hilus 3) Cortex 4) Medulla 5) Renal lobes 6) Medullary rays 7) Renal lobules |
|
|
The renal cortex contains (5)
|
1. Renal corpuscles
2. proximal tubules 3. distal tubules 4. peritubular capillaries 5. medullary rays |
|
|
8-18 conical medullary pyramids whose bases abut the corex and apices (renal papillae) are located in the
|
renal medulla
|
|
|
Renal medulla contains (4)
|
1) Medullary pyramids
2) collecting ducts 3) loops of Henle 4) vasa recta |
|
|
Renal papillae, cradled by a minor calyx, are perforated by openings of about
|
20 collecting ducts
|
|
|
Several minor calyces empty into a
|
major calyx, which empties into the renal pelvis, which drains into the ureter.
|
|
|
Portions of the renal cortex that extend between renal pyramids are called
|
renal columns
|
|
|
Each kidney has approximately how many lobes
|
8-18
|
|
|
Each renal lobe consists of
|
a medullary pyramid and associated cortex and numerous renal lobules
|
|
|
Medullary rays are what and are comprised of what?
|
extensions of ,edullary tissue into the cortex. clusters of collecting tubules and ducts.
|
|
|
One medullary ray occupies the center of each
|
renal lobule
|
|
|
Cortical material between medullary rays is know as the
|
labyrinth
|
|
|
Interlobular arteries and veins mark the borders between
|
adjacent renal lobules
|
|
|
highly convoluted structure that modified the fluid passing through it to form urine as it's final product
|
uriniferous tubule
|
|
|
Uriniferous tubule consists of two parts
|
nephron and collecting tubule
|
|
|
Approximately how many nephrons in each kidney
|
1.3 million
|
|
|
Several nephrons drain into a single
|
collecting tubule
|
|
|
multiple collecting tubules join in the deeper aspect of the medulla to form larger and larger
|
collecting ducts
|
|
|
Nephrons are located in the
|
cortical labyrinth surrounding the ray
|
|
|
# nephrons per collecting duct
|
10
|
|
|
Nephrons consist of 4 parts
|
1) Renal corpuscle
2) Proximal tubule 3) Loop of Henle 4) Distal tubule |
|
|
Each renal corpuscle consists of (3)
|
1) fenestrated capillaries
2) glomerulus 3) Bowman's capsule |
|
|
Bowman's capsule consists of what two layers
|
Visceral and parietal
|
|
|
The space between the visceral and parietal layers is known as
|
Bowman's space
|
|
|
The glomerulus is in intimate contact with which layer of Bowman's capsule
|
visceral
|
|
|
The glomerulus is supplied by 1) the short, straight ... and 2) drained by the ...
|
1) afferent glomerular arteriole
2) efferent glomerular arteriole |
|
|
The renal corpuscles and proximal and distal tubules are located in the ... while the loops of Henle are located in the ...
|
cortex
medulla |
|
|
Two types of nephrons are
|
1) cortical nephrons
2) juxtamedullary nephrons |
|
|
Characteristics of cortical nephrons
|
short, most common (86%), renal corpuscle in middle and outer portions of cortex, very short loop of Henle
|
|
|
Characteristics of JM nephrons
|
about 40 mm long, renal corpuscle is located in the cortex, but at the boundary of the cortex and medulla. Loop of Henle is long and projects into the medullary papilla
|
|
|
Kidneys receive approximately what percent of resting cardiac output?
|
20-25%
|
|
|
Approximate output of kidneys per day
|
1-2 liters
|
|
|
A branch of the abdominal aorta, divides into anterior and posterior segmental branches
|
Renal artery
|
|
|
Arise from the branches of the renal arteries in the renal hilus and penetrate the columns between the pyramids
|
Interlobar arteries
|
|
|
Branches of interlobar arteries at the corticomedullary junction
|
arcuate arteries which branch into interlobular arteries
|
|
|
Interlobular arteries branch to form these which drain into these
|
afferent arterioles
glomerular capillaries |
|
|
feeds the peritubular capillaries
|
efferent arteriole
|
|
|
Two capillary beds in the cortex connected by an arteriole
|
efferent arteriole from renal corpuscle of cortical nephrons supply peritubular capillaries in the cortex.
efferent arteriole from juxtamedullary nephrons supply vasa recta in the medulla |
|
|
The descending vasa recta carry isotonic blood into the medulla but the blood
|
loses water and picks up sodium as it passes deeper into the medulla
|
|
|
Unlike the loop of Henle, the ascending vasa recta are
|
permeable to salt and water.
|
|
|
Passive exchange of salt and water between the vasa recta and the interstitium is known as the
|
countercurrent exchange mechanism
|
|
|
The countercurrent exchange mechanism is important in
|
maintaining the osmotic gradient set up by the countercurrent multipler of Henle's loop
|
|
|
Blood in the ascending vasa recta drains into the
|
arcuate veins
|
|
|
Blood leaves the kidney through a single
|
renal vein and drains into the inferior vena cava
|
|
|
There are no anastomoses between interlobar arteries or between arcuate arteries. This, these arteries are examples of
|
end arteries
|
|
|
Each kidney receives 10% of the total blood volume per minute with >90% of this going to the
|
cortex
|
|
|
All the blood that reaches the medulla has already passed through the
|
cortex
|
|
|
Erythropoietin is manufactured and released by endothelial cells of the
|
peritubular capillary network
|
|
|
Most renal nerves original in the
|
celiac ganglion
|
|
|
Renin secretion is enhanced by
|
norepinephrine
|
|
|
Norepinephrine binds to what receptors in the afferent arteriole to cause vasoconstriction?
|
alpha-1-adrenergic
|
|
|
The fluid within Bowman's capsule is called the
|
glomerular filtrate
|
|
|
Glomerular filtrate contains
|
most inorganic ions and low molecular-weightorganic solutes (eg glucose and amino acids). Virtually no proteins and no cells.
|
|
|
The composition of the glomerular filtrate is altered by two general processes
|
tubular reabsorption and tubular secretion.
|
|
|
The tubule is all points intimately associated with the
|
peritubular capillaries
|
|
|
Glomerular capillaries lie between
|
afferent arteriole and efferent arteriole
|
|
|
In the glomerulus, blood filtering depends on three main pressures
|
1) blood hydrostatic pressure (promotes filtration)
2) capsillar hydrostatic pressure (oppose filtration) 3) Blood colloid asmotic pressure (oppose filtration) |
|
|
Net filtration pressure is
|
blood hydrostatic pressure minus capsular hydrostatic pressure and blood colloid osmotic pressure
|
|
|
Parietal layer of Bowman's capsule is composed of what type of epithelium?
|
simple squamous
|
|
|
Visceral layer of Bowman's capsule is composed of
|
podocytes
|
|
|
Characteristics of podocytes
|
long primary processes, interdigitate betweel glomerular capillaries. Slits between are covered by filtration slit diaphragm.
|
|
|
Bowman's space contains
|
urinary filtrate (not urine)
|
|
|
What extends from the urinary pole of the renal corpuscle?
|
proximal tubule
|
|
|
What is the entrance of afferent and exit of efferent arterioles?
|
Vascular pole
|
|
|
The glomerular basement membrane is actually
|
fused basal lamina (one central lamina densa sandwiched between two lamina rara.
|
|
|
Lamina rara contain what proteoglycan
|
heparan sulfate
|
|
|
Lemina densa contains what collagen type?
|
IR
|
|
|
Glomerular basement membrane is the site of
|
blood-urine barries in the kidney
|
|
|
Space between pedicels (processes of podocytes) is called the
|
filtration slit
|
|
|
What links adjacent pedicels?
|
filtration slit diaphragm
|
|
|
Sit in center of capillary loops, produce axial support for anastomosing glomerular capillaries. Sensitive to angiotensin II
|
Mesangial cells
|
|
|
Functions of mesangial cells
|
support of capillary loop system. Control blood flow through glomerular loops via angiotensin mechanism. Maintenance of glomerular basal lamina (phagocytosis of macromolecules lodged in GBM). Release and respond to growth factors. Able to divide
|
|
|
Begins at renal corpuscle's urinary pole. simple low columnar-to-cuboidal lining cells, tight junctions, brush border microvilli, basal infoldings.
|
proximal tubule
|
|
|
Reabsorption at the proximal tubule
|
100% of AA, glucose, small peptides, vitamins, HCO3-, 80% of sodium ions, 65% of water
|
|
|
Henle's loop: part which is permeable to salt and water
|
thin descending limb
|
|
|
Henle's loop: part which is impermeable to water
|
thick ascending limb
|
|
|
Extends from the proximal convoluted tubule in the cortex, dips into the medulla, and returns to the cortex where it empties into the distal convoluted tubule
|
Loop of Henle
|
|
|
Loop of Henle is a prerequisite for hypertonic urine and acts as a
|
countercurrent multiplier to establish an osmotic gradient in the interstitial fluid of the medulla
|
|
|
Tubular fluid delivered to the thin descending limb by the descending thick loop of Henle's loop is
|
isotonic, but proximal tubule reduces the volume from its raw state in Bowman's space
|
|
|
Maximum theoretical osmolarity is
|
1200 mOsm
|
|
|
Part of Henle's loop that has a more active role in setting up the gradient making medullary interstitium hypertonic
|
Ascending portion (impermeable to water). Contain Na/K/2Cl cotransporter in the apical membrane, which accounts for about 20% of the reabsorption of these ions from the tubular fluid.
|
|
|
Final segment of the nephron which lies in the cortex. Low cuboidal, no brush border. Folds of the basal plasma membrane
|
Distal tubule
|
|
|
Monitors MaCl concentration or flow rate of the tubular fluid
|
macula densa
|
|
|
Makes final adjustments of salt, water, and acid. Sensitive to aldosterone and ANP
|
distal convoluted tubule
|
|
|
Different embryologically from nephrons. Have distinct intercellular borders. Cuboidal in smaller tubules and columnar in larter ducts
|
Collecting ducts
|
|
|
Principle cells make up the
|
distal tubule
|
|
|
Helps to adjust pH in the collecting ducts
|
intercalated cells
|
|
|
Collecting ducts are under the influence of what hormone?
|
ADH
|
|
|
ADH causes
|
principle cells to become permeable to water through aquaporins.
|
|
|
Without ADH, urine is
|
hypotonic
|
|
|
Renal interstitium is
|
dense irregular collagenous connective tissue. Reticular and elastic fibers
|
|
|
Nerve fibers of the kidney are myelinated or unmyelinated
|
unmyelinated (sympathetic from the renal plexus)
|
|
|
papillary ducts are also known as the
|
ducts of Bellini
|
|
|
Area cribosa is the
|
sieve-like region near the ducts of Bellini
|
|
|
The portion of the apex of the pyramid that projects into the minor calyx is covered by
|
transitional epithelium (forms osmotic barrier that protects the surrounding tissues from the hypertonic urine and the urine from dilution)
|
|
|
Urine is propelling to the major calyx by
|
smooth muscle in lamina propria
|
|
|
In order to be filtered, a molecule must be
|
<69,000 daltons
not carry a high negative charge |
|
|
Chief barrier to negatively charged macromolecules is
|
heparan sulfate
|
|
|
Hypertonicity of the medullary interstitium is due to
|
Na, Cl, and urea
|
|
|
Responds to hypoxia by releasing EPO
|
EPO-producing cell
|
|
|
Cells that respond to a decrease in renal arterial blood flow are
|
juxtaglomerular cells (release renin when blood volume is decreased)
|
|
|
Juxtaglomerular apparatus is located
|
near each renal corpuscle's vascular pole
|
|
|
Juxtaglomerular apparatus includes
|
juxtaglomerular cells, macula densa, and lacis cells (mesangial cells)
|
|
|
JG cells are
|
modified smooth muscle cells in the afferent arteriole's wall. Source of renin (proteolytic enzyme)
|
|
|
Angiotensis I is converted to angiotensin II by
|
angiotensin-converting enzyme (ACE) in the lungs
|
|
|
function of angiotensin II
|
vasoconstrictor, increases blood pressure, stimulates aldosterone production by adrenal cortex thereby increasing Na+ and Cl- reabsorption by the distal tubule.
|
|
|
Angiotensin II inhibits
|
renin release
|
|
|
Lumen of ureters are lined by
|
transitional epithelium
|
|
|
Muscularis of ureters are
|
inner circular and outer longitudinal
|
|
|
Urinary bladder is composed of
|
transitional epithelium
|
|
|
During bladder distention, large round dome-shaped cells become stretched and change their morphology to become
|
flattened
|
|
|
These help to maintain strong cohesion between epithelials of bladder
|
Desmosomes
|
|
|
Reduce leakage in urinary bladder (form blood-urine barrier in the urinary bladder)
|
tight junctions
|
|
|
Triangular region of the bladder whose mucosa is always smooth
|
trigone
|
|
|
Muscularis of the bladder is also called the
|
detrusor muscle. Thin inner longitudinal, thick middle circular, thin outer longitudinal. Middle circular layer forms the internal sphincter.
|
|
|
Male urethra has 3 main parts
|
1) prostatic (transitional epi)
2) membranous (stratified columnar or pseudostratified epi) - skeletal muscle for external sphincter 3) penile (stratified columnar or pseudostratified. Patches of squamous) |
|
|
Female urethra is
|
shorter. carries only urine. lined by stratified squamous with patches of pseudostratified columnar. External sphincter is formed by the urogenital diaphragm.
|
|
|
Starling equation
|
P(Filtration) = P(capillary) - P(interstitial) - pi(capillary) - pi(interstitial)
P = hydrostatic pressure pi = oncotic pressure |
|
|
Hydrostatic pressure in capillaries depend on constriction of
|
precapillary sphincters
|
|
|
Nephron includes
|
glomerulus, proximal tubule, loop of Henle, distal tubule
|
|
|
Proximal tubule primarily absorbs
|
solutes and water
|
|
|
Loop of Henle
|
absorbs solute and aid further water absorption
|
|
|
distal tubule and collecting duct
|
control final solute absorption and contribute to acid/base balance.
|
|
|
The kidney has a portal system of what two capillary beds in series?
|
glomerular (high hydrostatic pressure) and peritubular capillaries (low hydrostatic pressure)
|
|
|
arteriole between the glomerular and pertubular capillaries
|
efferent arteriole
|
|
|
In the kidney, the filtered fluid has to pass through
|
the epithelium of the nephron before returning to the curculation
|
|
|
Net filtration in the glomerular capillaries is how much higher than the amount filtered by all other capillaries combined?
|
6 fold
|
|
|
Filtration rate per 100g tissue is how much more in the kidneys than the rest of the body?
|
10,000X
|
|
|
Glomerular filtrate passes through 3 layers
|
endothelium, basement membrane, and filtration slits.
|
|
|
Effective pore size of the glomerulus
|
3-5 nm
|
|
|
Rate-limiting barrier in filtration is formed by the
|
basement membrane
|
|
|
The presence of negatively charged proteoglycans (heparan sulfate) reduces the effective pore size for
|
anions
|
|
|
Forces driving filtration are the same as in systemic capillaries, except for the absence of a significant
|
oncotic pressure in Bowman's space
|
|
|
Oncotic pressure of the glomerular capillaries rises because of the increase in plasma protein concentration resulting from the large
|
filtration fraction
|
|
|
What percent of water is removed in glomerular capillaries? Systemic capillaries?
|
20%
<0.1% |
|
|
Glomerular filtration rate is determined by
|
hydraulic conductivity, area, hydrostatic pressure of capillaries, hydrostatic pressure of Bowman's space, and oncotic pressure of capillaries
|
|
|
2 ways filtration pressure can be increased
|
1) lowering the resistance of the afferent arteriole
2) increasing the resistance of the efferent arteriole |
|
|
The low permeability of the tight junction enables tight epithelia to maintain
|
large gradients of ion concentration and osmolarity
|
|
|
A specific inhibitor of Na/K pumps, which blocks fluid absorption across renal tubule cells. (Can also be blocked by lowering temperature)
|
ouabain
|
|
|
The primary driving force of fluid reabsorption is
|
active Na+ absorption
|
|
|
Transport of Na+ requres
|
uptake across the apical membrane AND extrusion across the basolateral membrane
|
|
|
Sugars, amino acids, peptides, phosphate, carboxylic acids are cotransported using
|
Na+ gradient created by Na/K pumps
|
|
|
In proximal tubule cells, glucose is taken up by
|
Na+:monosaccharide cotransporters (SGLT2) which can be inhibited by phlorizin.
|
|
|
Normal glucose plasma concentration
|
5mM
|
|
|
Renal threshold for glucose
|
10mM
|
|
|
Renal diabetes mellitus is characterized by
|
glucosuria despite normal plasma glucose levels
|
|
|
Renal diabetes mellitus is a reduced ability
|
to absorb glucose in the proximal tubule
|
|
|
The thick ascending limb of Henle's loop actively absorbs
|
Cl- (through Na+:K+:2Cl-cotransporters and K+channels). Na absorption through paracellular pathways. Driving force through Na+/K+ pump.
|
|
|
Na+:K+:2Cl- cotransporter can be inhibited by
|
furosemide (Lasix)
|
|
|
The thick ascending limbs are impermeable to
|
water (resulting in marked hypotonicity of the tubular fluid). Refered to as the diluting segment.
|
|
|
Na+ reabsorption in the early distal tubule proceeds bia
|
electroneutral Na+:Cl--cotransporters. Inhibited by thiazide diuretics.
|
|
|
Na+ absorption in the late distal tubule and collecting duct is
|
electrogenic, involving apical membrane Na+ channels.
|
|
|
Two types of cells are present in the colecting duct
|
Principle cells and intercalated cells
|
|
|
These cells actively absorb Na+ and secrete K+
|
Principle cells
|
|
|
These cells secrete hydrogen ions or bicarbonate, depending on acid-base status
|
intercalated cells
|
|
|
Na+ reabsorption by the principle cells is inhibited by
|
amiloride (block apical Na+ channels)
|
|
|
Absorption of Na+ in the late distal tubule and collecting duct is regulated by
|
aldosterone (stimulates Na+ transport by increasing the number of Na+ channels and Na+/K+ pumps)
|
|
|
Sustained plasma pH outside of this range is incompatible with life.
|
7.0-7.8
|
|
|
90% of bicarbonate is reabsorbed in the
|
proximal tubule (with the remaining absorbed in the late distal tubule and collecting duct)
|
|
|
Bicarbonate absorption in intercalated cells is driven by
|
proton pumps in the apical membrane, which create a large pH gradient between the cell and tubule lumen.
|
|
|
Exit of bicarbonate across the basolateral membrane is mediated by an
|
anion exchanger
|
|
|
pH of the final urine reaches values as low as
|
4.5
|
|
|
Normal production of nonvolatile acids in the body
|
60 millimoles
|
|
|
For human plasma,
1) pK' = ? 2) [HCO3-] = ? 3) Pco2 = ? 4) Solubility coefficient of CO2 |
1) 6.1
2) 24mM 3) 40 mmHg 4) 0.03 mM/mmHg |
|
|
pH of normal blood plasma is
|
7.40
|
|
|
Metabolic acid/base disorders
|
ketoacidosis (diabetes)
lacticacidosis (exercise, hypoxia) bulemia (alkalosis) |
|
|
Respiratory acid/base disorders
|
COPD (acidosis)
hyperventilation (alkalosis) |
|
|
Step 1 - Determine if the blood is alkaline or acid
|
Normal range is 7.38-7.42
|
|
|
Step 2 - Determine if the main disorder is respiratory or metabolic.
|
If pH INVERSES pCO2, then it's respiratory
If pH FOLLOWS pCO2, then it's metabolic. |
|
|
Step 3 - ONLY FOR RESPIRATORY - is it acute or chronic?
|
(acute) dpH = 0.08 (Pco2 - 40)/10
(chronic) dpH = 0.03 (Pco2 - 40)/10 |
|
|
Steps 4,5,6 are for
|
METABOLIC ONLY
|
|
|
Step 4 - Determine nature of a metabolic acidosis by calculating anion gap
|
anion gap = [Na] - [Cl] - [HCO3-]
|
|
|
Normal value for [Na]
|
140 mM
|
|
|
Normal value for [Cl]
|
104 mM
|
|
|
Normal value for [HCO3]
|
24 mM
|
|
|
Normal anion gap
|
12+-2 mM
|
|
|
Step 5 - Is the respiratory compensation of a metabolic disorder appropriate?
|
Pco2 (normal range) = [1.5(HCO3-)]+(8+-2)
|
|
|
Step 6 - Does the anion gap explain the whole dHCO3-?
|
Normal HCO3- = 24mM => HCO3-(actual) + (AG-12)
|
|
|
Default phenotypic sexual development is
|
femail
|
|
|
Two functions of the ovaries
|
gametogenesis and steroidogenesis
|
|
|
Production of gametes (female) is called
|
oogenesis
|
|
|
Developing gametes (female) are called
|
oocytes
|
|
|
Mature gametes (female) are called
|
ova
|
|
|
These hormones promote growth and maturation of the internal and external sex organs (female) and are responsible for the female secondary sexual characteristics that develop at puberty.
|
Estrogens (17-beta-estradiol, estrone, estriol)
|
|
|
Estrogens and progesterone both work on
|
brests (Progesterone -> Alveoli, Estrogen -> Ducts)
|
|
|
Prepare the internal sex organs, mainly the uterus, for pregnancy by promoting secretory changes in the endometrium. Also prepares the mammary gland for lactation by promoting proliferation of the mammary gland alveoli
|
Progesterone
|
|
|
The surface epithelium covering the ovaries is called the
|
germinal epithelium (low cuboidal)
|
|
|
Directly beneath if germinal epithelium is the
|
tunica albuginea. Poorly vascularized, dense irregular collagenous capsule. No septae or trabeculae.
|
|
|
The primordial germ cells are derived from
|
endoderm
|
|
|
This gives rise to almost three fourths of all ovarian tumors
|
germinal epithelium
|
|
|
True or False. The ovary has no ducts.
|
True
|
|
|
The ovary consists of three regions
|
Cortex, Medulla, Hilus
|
|
|
The most prominent cortical structures are
|
oocyte-containing ovarian follicles
|
|
|
Contains loose connective tissue, a mass of relatively large coiled blood vessels, lymphatic vessels, and nerves. Ovarian follicles are absent.
|
Medulla
|
|
|
Provide the microenvironment for the developing oocyte
|
ovarian follicles
|
|
|
Early stages of oogenesis occur during fetal life when mitotic divisions massively increase the number of
|
oogonia
|
|
|
The oocytes present at birth remain arrested in development at the
|
first meiotic division (dictyotene stage of meiosis I).
|
|
|
True or False. There are no new oocytes after birth.
|
True
|
|
|
Most of the 400,000 primary oocytes present at menarche do not complete maturation and are gradually lost through
|
atresia
|
|
|
atresia is mediated by
|
apoptosis of cells surrounding the oocyte
|
|
|
Everything in the ovary is derived from ... except for oocytes which is derived from ...
|
mesoderm
endoderm |
|
|
Follicular cells are derived from
|
mesothelial epithelium
|
|
|
Primary oocyte is derived from
|
endoderm
|
|
|
Primordial follicles are composed of a single layer of
|
squamous follicular cells that surround the primary oocyte
|
|
|
Primordial follicles are separated from the ovarian stroma by a
|
basement membrane
|
|
|
Organelles of the oocyte include
|
single nucleolus, numerous mitochondria, abundant Golgi, rER but with few ribosomes, occasional annulate lamellae.
|
|
|
The nucleus of the primary oocyte becomes suspended in meiosis at the
|
diplotene stage of the first meiotic division. The suspended state is called dictyotene.
|
|
|
The nucleus of the primary oocyte contains
|
46 chromosomes (4N)
|
|
|
The flattened follicular cells completely surround the primary oocyte and are attached to each other by
|
desmosomes, gap junctions. NO TIGHT JUNCTIONS.
|
|
|
A follicle with only a single layer of cuboidal follicular cells encircling the oocyte
|
unilaminar primary follicle
|
|
|
A follicle with several layers of cells around the primary oocyte
|
multilaminar primary follicle (granulosa cells, collectively form the stratum granulosum, an avascular epithelium)
|
|
|
Grows to about 100 to 150 micrometers in diameter with an enlarged nucleus. Several Golgi, rER becomes rich with ribosomes, free ribosomes are abundant, and mitochondria are numerous. Numerous cortical granules containing hydrolytic enzymes are located in the oocyte's cytoplasm just below the plasma membrane
|
primary oocyte
|
|
|
Proliferative activity of granulosa cells is due to
|
activin, produced by the primary oocyte
|
|
|
An amorphous substance which separates the oocyte from the surrounding follicular cells, composed of three different glycoproteins, ZP1, ZP2, and ZP3
|
zona pellucida
|
|
|
Serves as the sperm receptor
|
ZP3
|
|
|
Oocyte and follicular cells communicated with each other through
|
gap junctions formed between microvilli of oocyte and filopodia of the follicular cells
|
|
|
Stromal cells begin to be organized around the multilaminar primary follicle, formin an inner
|
theca interna, composed mostly of a richly vascularized cellular layer
|
|
|
The theca cells produce an angiogenesis factor that promotes
|
development of blood vessels
|
|
|
The cells composing the theca interna posses
|
LH receptors in their plasma membranes
|
|
|
Theca interna cells produce
|
androstenedione which enters the granulosa cells, where it is converted by the enzyme cytochrome P450 aromatase into estradiol
|
|
|
Several intercellular spaces develop within the mass of granulosa cells and become filled with a fluid known as
|
follicular fluid
|
|
|
Once a multilaminar primary follicle displays the presence of follicular fluid, it is known as a
|
secondary follicle
|
|
|
Granulosa cells develop plasma membrane receptors for
|
FSH
|
|
|
Continued proliferation of the granulosa cells of the secondary follicle depends of
|
FSH released by basophils of the anterior pituitary
|
|
|
As more fluid is produced, individual droplets of follicular fluid coalesce to form a signle, fluid-filled chamber called
|
the antrum
|
|
|
7 layers of a mature Graafian follicle
|
1) theca externa
2) theca interna 3) antrum 4) stratum granulosum 5) primary oocyte 6) cumulus oophorus 7) corona radiata |
|
|
These follicles undergo ovulation, with a diameter reaching 15-20 mm
|
Graafian follicles
|
|
|
Small group of granulosa cells that project out from the wall into the fluid-filled antrum that surrounds the oocyte is known as the
|
cumulus oophorus
|
|
|
Single layer of granulosa cells that immediately surrounds the primary oocyte is called the
|
corona radiata
|
|
|
Continued formation of liquor folliculi causes the cumulus oophorus composed of the primary oocyte, the corona radiata, and associated follicular cells to become
|
detached from its base to float freely within the liquor folliculi.
|
|
|
Each menstrual cycle stimulates approximately how many follicles?
|
20-40
|
|
|
FSH from the anterior pituitary binds to
|
granulosa cells
|
|
|
granulosa cells are stimulated to
|
divide, form cytochrome p450 aromatase, which converts androstenedione to estradiol, stimulate gap junction and LH receptor formation
|
|
|
LH from the anterior pituitary binds to receptors on the
|
theca interna cells
|
|
|
Theca interna cells form
|
androstenedione, which diffuses across the basement membrane of the follicle into granulosa cells where it is converted to estradiol
|
|
|
Estrogen synthesis by ovarian follicles involves two cell types
|
theca interna cells and granulosa cells
|
|
|
Effects of estradiol in the ovary (2)
|
1) increase mitosis of granulosa cells
2) stimulates formation of LH receptors on granulosa cells |
|
|
Estradiol is carried to the anterior pituitary where it increases its sensitivity to
|
GnRH, which results in the LH surge that induces ovulation.
|
|
|
The LH surge causes (3)
|
1) release of meiosis-inducing substance (maturation promoting substance)
2) MIS(MPS) causes the Graafian follicle to complete its first meiotic division, resulting in secondary oocyte and first polar body 3) Newly formed secondary oocyte enters the second meiotic division and is arrested in metaphase |
|
|
The process of releasing the secondary oocyte from the Graafian follicle is known as
|
ovulation
|
|
|
By the 14th day of menstruation, elevated blood estrogens cause (2)
|
1) Negative feedback inhibition shuts off FSH release by the anterior pituitary
2) Surge of LH released by basophils of the anterior pituitary |
|
|
Several hours prior to ovulation, 7 things occur
|
1) Cumulus mass breaks up
2) Primary oocyte becomes secondary oocyte 3) Follicular fluid accumulates in the antrum, but NO increase in pressure. 4) Increased blood flow to ovaries results in edema (histamine, prostaglandins, and collagenase released near follicle) 5) Surface of ovary near follicle loses blood supply (stigma) 6) Connective tissue at stigma degenerates 7) Stigma ruptures releasing secondary oocyte and cumulus mass into oviduct |
|
|
Ovulation is always on the
|
14th day BEFORE the BEGINNING of menstruation
|
|
|
Luteinization is the convertion of
|
remnants of the Graafian follicle into the corpus hemorrhagicum then into the corpus luteum.
|
|
|
Stages of fertilization and time frames
|
1) Fertilization (24-48 hours after ovulation)
2) Morula in uterine cavity (2-3 days after fertilization) 3) Implantation (6-7 days after fertilization) |
|
|
Corpus hemorrhagicum is the
|
remainder of the Graafian follicle plus blood clot
|
|
|
Blood clot is removed from the corpus hemorrhagicum and LH converts it into
|
corpus luteum
|
|
|
Corpus luteum functions as an
|
endocrine gland
|
|
|
Corpus luteum is composed of
|
granulosa lutein cells (modified granulosa cells) and theca lutein cells (modified theca interna cells)
|
|
|
80% of the cell population of the corpus luteum
|
granulosa lutein cells
|
|
|
Granulosa lutein cells mostly produce
|
progesterone and convert androgens produced by the theca lutein cells into estrogens
|
|
|
Progesterone stimulates growth and secretory activity of the
|
uterine endometrium, preparing it for implantation.
|
|
|
The theca interna cells become modified into hormone-secreting cells known as
|
theca lutein cells
|
|
|
Theca lutein cells specialize in the production of
|
estrogens (some progesterone and androgens)
|
|
|
Progesterone and estrogens inhibit the secretion of
|
LH and FSH (prevents second ovulation)
|
|
|
If pregnancy, hCG maintains the corpus luteum for 3 months. hCG is secreted by the
|
placenta
|
|
|
If pregnancy does not occur, absence of hCG leads to degeneration of the
|
corpus luteum
|
|
|
Inherent lifespan of the corpus luteum
|
14 days
|
|
|
Corpus luteum is invaded by fibroblasts, becomes fibrotic, ceases to function, undergoes luteolysis and is phagocytosed by macrophages. Fibrous connective tissue forms in its place and is known as the
|
corpus albicans
|
|
|
Once a single mature follicle ruptures and releases its secondary oocyte and associated cells, the remaining follicles
|
undergo atresia and the resulting atretic follicles are eventually phagocytized by macrophages
|
|
|
Of all of the follicles present in the ovaries at menarche, what percentage develop to maturity and undergo ovulation?
|
0.1-0.2%
|
|
|
Last structure to be broken down during atresia
|
zona pellucida
|
|
|
The incidence of atresia is greatest in the
|
least mature follicles
|
|
|
The medulla of the premenstrual ovary has interstitial cells that secrete ... and hilus cells that secrete ...
|
estrogens
androgens |
|
|
Oviducts are suspended by a thin mesentery known as the
|
mesosalpinx
|
|
|
Mesosalpinx is derived from the
|
broad ligament
|
|
|
4 regions of the oviduct
|
1) infundibulum (with fimbriae)
2) ampulla (site of fertilization) 3) isthmus 4) intramural part (within the wall of uterus) |
|
|
Lumen of the oviduct is lined by
|
simple columnar epithelium (some ciliated, some secretory)
|
|
|
2 cells founds in the oviduct epithelium
|
1) Ciliated cells - beat towards uterus
2) Peg cells - secretes nutrition for sperm and ovum |
|
|
Muscularis of oviduct (inner circular and outer longitudinal) propel oocyte via
|
peristaltic waves of contraction
|
|
|
Greatest amount of oviduct folding is in the
|
ampulla
|
|
|
Estrogen affects the oviduct and causes changes such ash
|
increased coliogenesis, secretory activity and height of the lining epithelial cells.
|
|
|
3 parts of the uterus
|
body, fundus, cervix
|
|
|
uterine wall of body and fundus is composed of
|
endometrium (serosa), myometrium (muscularis), and either adventitia or serosa
|
|
|
Outer serous layer or visceral peritoneum covering the uterus
|
perimetrium
|
|
|
Thicked subcompartment of the wall of the uterus
|
myometrium
|
|
|
Myometrial layers
|
Outer and inner longitudinal, plus richly vascular middle layer of circular (house arcuate arteries).
|
|
|
At the cervix, the myometrium is composed of
|
dense irregular connective tissue containing elastic fibers
|
|
|
Size and number of the myometrial muscle cells are related to
|
estrogen levels
|
|
|
Luminal surface of the endometrium is composed of
|
simple columnar (ciliated and nonciliated secretory)
|
|
|
In the endometrium, the epithelium forms simple tubular glands that invaginate the endometrial stroma and extend as far as
|
the myometrium
|
|
|
The structure of the endometrial glands vary as the endometrium pass through the phases of
|
the menstrual cycle
|
|
|
Subdivisions of the endometrium
|
stratum functionalis - structural changes during menstruation (lost)
stratum basalis - unaffected by menstruation (cell source) |
|
|
Dual blood supply of the endometrium
|
Spiral arteries - supply functional layer
straight arteries - supply basal layer |
|
|
The presence of endometrial tissue in the pelvis or in the peritoneal cavity is known as
|
endometriosis
|
|
|
Cervical canal lined by
|
mucus-secreting simple columnar epithelium
|
|
|
Cervical glands may become blocked and form
|
Nabothian cysts (not pathological)
|
|
|
This luteal hormone induces lysis of collagen in the cervical walls during parturition.
|
relaxin
|
|
|
During menstruation, cervical epithelium is
|
unaffected. However, mucus consistency changes during the cycle.
|
|
|
Cervical mucus is water, serous fluid during
|
proliferative phase
|
|
|
Cervical mucus is more viscous during
|
secretory phase and pregnancy
|
|
|
Hormone that regulates the changes in the viscosity of the cervical gland secretions
|
Progesterone
|
|
|
Abrupt transition between simple columnar and stratified squamous nonkeratinized epithelium at the
|
cervical-vaginal junction
|
|
|
Cervical carcinoma develops from
|
stratified squamous epithelium of the cervix
|
|
|
Three phases of the menstrual cycle
|
1) Proliferative - secretion of estrogen by growing follicles. Corresponds to follicular phase of the ovary
2) Secretory - secretion of progesterone by the corpus luteum. Corresponds to the luteal phase of the ovary 3) Menstrual - decreased hormone production and decline in the corpus luteum. |
|
|
Proliferative phase
|
days 5-14 (most variable). Functional layer becomes much thicker (MITOSIS of the surface endometrial epithelium and stroma).
|
|
|
During the proliferative, glycogen accumulates
|
in the basal cytoplasm of the glandular epithelial cells
|
|
|
Ovulation occurs on day
|
14 of a 28 day cycle
|
|
|
The secretory phase
|
(days 15-24) begins the day after ovulation and is less variable in duration. Divided into early and late stage. Corresponds to the luteal phase of the ovary.
|
|
|
The uterus is prepared for implantation during the
|
Early secretory phase
|
|
|
Most of the increase of the endometrium in the secretory phase is due to
|
edema
|
|
|
During early secretory phase, endometrial glands have a
|
tortuous SACCULATED appearance, due to the accumulation of secretions.
|
|
|
In the early secretory phase, glycogen accumulates in the glandular epithelial cells
|
basal cytoplasm, but then shifts to the apical cytoplasm.
|
|
|
Late secretory (premenstrual) phase
|
(day 25-28) AKA ischemic phase. Endometrium shrinks due to loss of interstitial fluid. Blow flow is impaired causing necrosis of the functional layer
|
|
|
Menstrual phase
|
(days 1-4). Reduced levels of progesterone and estrogen.
|
|
|
During menstruation, approximately how much blood is lost?
|
35mL
|
|
|
Vaginal discharge during menstruation consists of
|
blood, uterine fluid, and sloughing stromal and epithelial cells from the stratum functionalis.
|
|
|
Blood clotting during menstruation is inhibited by
|
fibrinolysin
|
|
|
Disintegration of the endometrium appears to be the result of impairment of its blood supply that is closely related to
|
decreased progesterone secretion by the degenerating corpus luteum
|
|
|
A solid mass of ~16 cells
|
morula
|
|
|
hollow ball of cells
|
blastocyst
|
|
|
Outer layer of trophoblast (fetal tissue)
|
syncytiotrophoblast
|
|
|
Function of syncytiotrophoblast
|
exchanges between maternal and fetal blood must occur across it
|
|
|
Simple cuboidal epithelium deep to the syncytiotrophoblast. Dividing cells of this contribute to the overlying syncytiotrophoblast
|
cytotrophoblast
|
|
|
Region of the chorion which is the fetal component of the placenta is called the
|
chorion frondosum
|
|
|
The layer of the placenta from which the villi project is called the
|
chorionic plate
|
|
|
Maternal blood flows through the intervillous spaces, and the blood is in contact with the
|
surface of villi (fetal villi-maternal blood interface)
|
|
|
Villi anchored to the decidua basalis are called
|
anchoring villi
|
|
|
Villi suspended in maternal blood of the lacunae are known as
|
free villi
|
|
|
Formed by the syncytiotrophoblast, cytotrophoblast and associated connective tissue
|
chorion
|
|
|
Placental villi arise from the
|
chorionic plate
|
|
|
Between the myometrium and developing embryo
|
decidua basalis
|
|
|
Exchange of gases and metabolites occurs between fetal and maternal blood across the
|
placental barrier (sinusoidal capillaries, no tight junctions, no paracellular route)
|
|
|
Cells and layers across which material must be transported (6)
|
1) syncytiotrophoblast
2) cytotrophoblast 3) basal lamina of the trophoblast 4) connective tissue of the villus 5) basal lamina of the placental capillary 6) endothelium of the capillary |
|
|
Molecules that passively diffuse across placenta
|
O2, CO2, fatty acids, steroids, electrolytes
|
|
|
Molecules that diffuse by facilitation across placenta
|
glucose
|
|
|
Molecules that are actively transported across the placenta
|
amino acids
|
|
|
Molecules that cross the placenta by receptor-mediated endocytosis
|
Insulin, IgG
|
|
|
Other molecules that can cross the placental barrier
|
viruses (rubella, HIV), alcohol, drugs
|
|
|
Placental hormones
|
hCG, progesterone, estrogens (with fetoplacental unit), human chorionic somatomammotropin (hCS) - growth-promoting and lactogenic hormone, human chorionic thyrotropin (hCT), relaxin, leptin
|
|
|
decidual cells enlarge and synthesize
|
prolactin and prostaglandins
|
|
|
Mucous tissue in the umbilical cord is referred to as
|
Wharton's jelly
|
|
|
Vaginal epithelium
|
stratified squamous nonkeratinized (langerhans cells present). Accumulate glycogen under the influence of estrogens.
|
|
|
Lactic acid in the vagina is formed by
|
vaginal bacterial flora
|
|
|
True or False. Vagina does not contain any glands.
|
True
|
|
|
Sexual stimulation increases vaginal secretions which is
|
derived from transudate from thin-walled veins in the lamina propria combined with secretions from cervical glands.
|
|
|
Vaginal muscularis consists of
|
inner circular and outer longitudinal smooth muscle, and a ring of skeletal muscle circles the opening of the vagina.
|
|
|
The vestibule between the labia minora receives secretions of the
|
Bartholin glands
|
|
|
True or False. Mammary glands are modified sweat glands.
|
True
|
|
|
There are 15-20 lobes in each gland and each lobe opens onto the apex of the nipple via
|
a lactiferous duct
|
|
|
Dilated lactiferous duct for milk storage
|
lactiferous sinus
|
|
|
Promotes the development of mammary ducts
|
estrogens
|
|
|
Promotes the development of secretory alveoli
|
progesterone
|
|
|
Milk contains
|
minerals, electrolytes, carbohydrates (including lactose), IgA, proteins (including caseins), and lipids
|
|
|
Two types of breast cancer
|
ductal carcinoma (80-90%) and lobular carcinoma
|
|
|
Milk ejection reflex is stimulated by (3)
|
1) mechanical stimulation (afferent impulses to hypothalamus)
2) Oxytocin (contraction of myoepithelial cells) 3) Prolactin (production of milk) |
|
|
Lipids in milk are secreted by
|
apocrine secretion
|
|
|
Proteins in milk are secreted by
|
merocrine secretion
|
|
|
interstitial cells of Leydig secrete
|
androgens (especially testosterone)
|
|
|
conversion of testosterone to DHT occurs via
|
5-alpha-reductase
|
|
|
Sertoli cells secrete
|
inhibin and androgen-binding hormone
|
|
|
Extension of the peritoneum carried through the inguinal canal by the descending testis which covers the anteriolateral surface of the testes.
|
tunica vaginalis
|
|
|
thick fibrous capsule of dense irregular collagenous connective tissue with some smooth muscle while covers the testis
|
tunica albuginea
|
|
|
The tunica albuginea thickens around the posterior side to form the
|
mediastinum testis
|
|
|
The inner surface of the capsule is a thin vascular loose connective tissue layer called the
|
tunica vasculosa
|
|
|
Each lobule of the testis contains 1-4 highly coiled
|
seminiferous tubules (each 30-70cm in length). In total about 0.3 miles of tubule dedicated to sperm production.
|
|
|
loose connective tissue between the seminiferous tubules contains small clusters of
|
interstitial cells of Leydig (plus fenestrated and lymphatic capillaries)
|
|
|
stratified epithelium surrounding the seminiferous tubules
|
seminiferous epithelium
|
|
|
true epithelial cells of the seminiferous tubules
|
sertoli cells
|
|
|
Everything in the testis is derived from mesoderm except
|
germ cells (endoderm)
|
|
|
Germ cell differentiation
|
Spermatogonia->primary spermatocytes->secondary spermatocytes->spermatid->sperm
|
|
|
Sertoli cells are resistant to
|
heat,x-rays,infection and malnutrition
|
|
|
Most numerous epithelial cells before puberty
|
Sertoli cells
|
|
|
Tall columnar epithelial cells that line the lumen of the seminiferous tubule
|
Sertoli cells
|
|
|
Sertoli cells have plasma membrane receptors for
|
FSH
|
|
|
Sertoli-germ cell junctions
|
desmosomes
|
|
|
Sertoli-basal lamina junctions
|
hemidesmosomes
|
|
|
Sertoli-Sertoli junctions
|
Gap and tight junctions
|
|
|
Difference in Sertoli-Sertoli tight junctions from regular tight junctions
|
independent of the zonula adherens, includes more than 50 parallel fusion lines in adjacent membranes, stimulated by FSH.
|
|
|
Sertoli-Sertoli tight junctions setup 2 compartments
|
1) Basal - cell are in mitosis and early stage of meiosis
2) Adluminal - meiosis and spermiogenesis (primary and secondary spermatocytes and spermatids are restricted to this compartment) |
|
|
"Passing through" tight junctions by the primary spermatocytes is accomplished by
|
a tight junction forming on the basal side of the spermatocyte while the apical tight junction breaks down.
|
|
|
Sertoli-Sertoli tight junctions also form
|
blood-testis barrier
|
|
|
The adluminal compartment contains a high concentration of
|
ABP (and therefore testosterone). Important for gamete development.
|
|
|
Functions of Sertoli cells
|
1) Blood-testis barrier
2) Supporting cells - metabolic exchange medium for germ cells 3) Phagocytic - residual bodies and failed germ cells 4) Exocrine (testicular fluid, transferrin, ABP) and endocrine (inhibin, anti-mullerian hormone in embryo) |
|
|
True or False. Prior to puberty, only spermatogonia exist.
|
True
|
|
|
Meiosis begins...
|
at puberty
|
|
|
The process of producing sperm requires
|
FSH, LH, prolactin, testosterone. 64 days
|
|
|
Three phases of spermatogenesis
|
1) spermatogonial phase (spermatocytogenesis)
2) spermatocyte phase (meiosis) 3) spermatid phase (spermiogenesis) |
|
|
What germ layer are spermatogonia derived from?
|
Endoderm
|
|
|
Three types of spermatogonia
|
1) Type A dark spermatogonia (reserve cells)
2) Type A pale spermatogonia (replicating population) 3) Type B spermatogonia |
|
|
Type A dark cells can give rise to
|
Type A dark and type A pale cells (self-renewing)
|
|
|
Type A pale cells are
|
true stem cells induced by testosterone (ABP) to proliferate
|
|
|
Type A pale cells produced from the division of a type A dark cell remains
|
attached to one another by a cytoplasmic bridge (likewise with daughters of subsequent mitosis)
|
|
|
After several divisions, type A pale cells differential into
|
type B cells
|
|
|
Type B cells are
|
progenitor cells (non-self-renewing), differentiate into primary spermatocytes.
|
|
|
haploid number of primary spermatocyte
|
4N
|
|
|
Primary spermatocytes remain in the
|
adluminal compartment
|
|
|
DNA composition of type A pale spermatogonia
|
46 (2N)
|
|
|
DNA composition of type B spermatogonia
|
46 (4N)
|
|
|
DNA composition of primary spermatocyte after first meiotic division
|
23 (2N)
|
|
|
DNA composition of secondary spermatocyte after second meiotic division
|
23 (1N)
|
|
|
Process by which early spermatids differentiate into late spermatids
|
spermiogenesis
|
|
|
During the transformation of spermatids into sperm
|
accumulate hydrolytic enzymes, rearrange and reduce the number of organelles, form flagella, shed some cytoplasm
|
|
|
4 phases of spermiogenesis
|
1) Golgi phase
2) cap phase 3) acrosomal phase 4) maturation phase |
|
|
Where to morphological changes of spermatids occur?
|
while the spermatids are embedded in invaginations of the luminal surface of the Sertoli cells
|
|
|
During the golgi phase, hydrolytic enzymes are formed in the
|
rough ER, modified by Golgi, packaged by trans-golgi->small, membrane bound, PAS-positive granules called proacrosomal granules
|
|
|
What determines the anterior pole in the developing sperm?
|
acrosomal vesicle
|
|
|
The cap phase of spermiogenesis is characterized by
|
the formation of the acrosomal cap, and the migration of the paired centrioles from the juxtanuclear region to the newly established posterior pole of the spermatid where it initiates synthesis of the axoneme of the flagellum.
|
|
|
During the acrosomal phase
|
the spermatid reorients itself so that the head points toward the basal lamina of the seminiferous tubule. The centrioles return to attach to the posterior nucleus, and form the neck of the spermatid.
|
|
|
The maturation phase of spermiogenesis is characterized by
|
pinching off of the excess cytoplasm as residual bodies that are phagocytized by Sertoli cells. The cytoplasmic bridges that have characterized the developing gametes since the type A pale spermatogonia remain with the residual bodies.
|
|
|
The mature spermatids are released from the surface of the Sertoli cells into the lumen of the seminiferous tubule which is a process known as
|
spermiation (100 million per day per testis)
|
|
|
The middle piece of the sperm tail is filled with
|
mitochondria
|
|
|
The acrosome covers the anterior two-thirds of the nucleus. The acrosome is a highly specialized
|
lysozome.
|
|
|
The acrosome contains enzymes including
|
hyaluronidase, acid phosphatase, and a trypsin-like protease known as acrosin.
|
|
|
Binding of a sperm to the 1)... molecule of the zona pellucida triggers the 2)
|
1) ZP3
2) acrosomal reaction |
|
|
Transport of the newly released sperm (nonmotile)
|
seminiferous tubules->straight tubules->rete testis->efferent ductules->epididymal duct.
|
|
|
Most of the fluid secreted in the seminiferous tubules is reabsorbed in the
|
efferent ductules
|
|
|
Sperm develop motility as they poass through the 4-5 meters of the highly coiled
|
epididymal duct
|
|
|
Sperm are propelled to the distal portion of the epididymal duct by
|
peristaltic contractions of smooth muscle that surrounds the efferent ductules
|
|
|
Where are sperm stored before ejaculation?
|
distal portion of the epididymal duct
|
|
|
Mature sperm can live for ... in the male genital duct system
|
several weeks
|
|
|
Sperm only survive ... in the female reproductive system
|
2-3 days
|
|
|
Sperm acquire the ability to fertilize an ovum only after existing some time in the female tract, by a process called
|
capacitation
|
|
|
6 factors that affect spermatogenesis
|
1) dietary deficiencies
2) infections 3) administered hormones 4) toxins 5) irradiation 6) elevated testicular temperature |
|
|
Temperature within the scrotum
|
2-3 degrees C below body temp (essential for spermatogenesis)
|
|
|
Blood supply to the testis is through the
|
testicular artery
|
|
|
Testicular artery is surrounded by the
|
pampiniform plexus of veins which forms a countercurrent heat exchanger
|
|
|
Spermatic chord is made up by
|
artery, veins, nerves, lymphatics, and the vas deferens
|
|
|
Cremaster muscle is continuous with the
|
internal oblique
|
|
|
Leydig cells (Nondividing in adult) are derived from
|
mesoderm
|
|
|
Leydig cells differentiate early in fetal life and secrete
|
testosterone
|
|
|
At puberty, Leydig cells are exposed to
|
LH stimulation (from anterior pituitary) and again differentiate into androgen-secreting cells that produce testosterone.
|
|
|
In the adult, secretion of testosterone is essential for the
|
maintenance of spermatogenesis
|
|
|
Leydig cells are acidophilic. What accounts for this?
|
They are hormone-secreting, so they have numerous lipid droplets, mitochondria with tubular cristae, and an elaborate smooth ER.
|
|
|
There are no secretory granules in the Leydig cells because...
|
testosterone is released constituitively
|
|
|
GnRH from the hypothalamus binds to anterior pituitary basophils stimulating the release of
|
LH.
|
|
|
LH is carried to the testis in the blood stream and binds to
|
LEYDIG CELLS (causes testosterone production, negative feedback to hypothalamus and anterior pituitary)
|
|
|
Testosterone stimulates
|
SERTOLI CELLS, which is a requirement for support of sperm production (also secondary sex characteristics).
|
|
|
Prolactin binds to
|
LEYDIG CELLS and enhances the stimulatory effect of LH on Leydig cells
|
|
|
GnRH from the hypothalamus stimulates release of
|
FSH from the anterior pituitary basophils.
|
|
|
FSH exclusively binds to
|
SERTOLI CELLS to stimulate ABP production.
|
|
|
ABP binds and concentrates
|
androgens in the lumen of the tubules
|
|
|
FSH also stimulates SERTOLI CELLS to produce
|
inhibin, which inhibits the FSH release from the anterior pituitary.
|
|
|
Intratesticular ducts include
|
straight tubules, rete testis, and proximal portion of efferent ductules.
|
|
|
First half of the straight tubules is solely lined by
|
Sertoli cells
|
|
|
Second half of the straight tubules is lined by
|
simple cuboidal epithelium
|
|
|
A complex series of interconnecting channels in the highly vascular connective tissue of the mediastinum testes
|
rete testis (low cuboidal epithelium)
|
|
|
Approximately 15 short efferent ductules leave the testis by penetrating the tunica albuginea and connect the rete testis to the proximal portion of the
|
epididymal duct
|
|
|
Efferent ductules are lined with two types of cells
|
1) tall columnar ciliated
2) short cells (w/ microvilli) |
|
|
Amount of testicular fluid reabsorbed in the efferent ductules
|
95% (this causes a downstream current which moves sperm)
|
|
|
Contraction of the fibromuscular coat help to move sperm from the efferent ductules to the
|
epididymis
|
|
|
distal portions of the efferent ductules, epididymal duct, vas deferens, ejaculatory duct, and urethra make up the
|
excurrent duct system
|
|
|
The epididymal duct is a very long, highly coiled tube in which sperm undergo maturation, and is contained within the
|
epididymis
|
|
|
The coiled epididymal duct measures
|
4-6 meters in length
|
|
|
The epididymal duct is lined with
|
pseudostratified epithelium, consisting of tall columnar principal cells and short basal cells.
|
|
|
Basal cells are
|
stem cells, synthesize glutathione S-transferase
|
|
|
Principal cells are characterized by
|
long, apical stereocilia
|
|
|
Principal cells are secretory with well-developed rough ER, large Golgi. They secrete
|
1) sialic acid and glycoproteins - added to sperm glycocalyx
2) organic acids - keep sperm nonmotile 3) glycerophosphocholine - inhibits capacitation |
|
|
Principle cells phagocytize
|
any sperm that degenerate in the duct
|
|
|
Principle cells absorb
|
HCO3-, helps to keep a low lumen pH
|
|
|
Smooth muscle of the epididymis
|
1) Head and body - thin layer of circular muscle
2) Tail - inner longitudinal, middle circular, outer longitudinal = continuous with vas deferens |
|
|
Sperm entering the head of the epididymis are not capable of fertilization, but
|
once they reach the tail where they are stored, they are.
|
|
|
In the head region, the smooth muscle is not innervated (spontaneous contractions), but in the tail region, the smooth muscle becomes heavily innervated by
|
sympathetic fibers (all muscle cells are directly innervated)
|
|
|
Vas deferens connects to which part of the urethra?
|
Prostatic
|
|
|
The thick smooth muscle of the vas deferens is composed of what three layers? Innervation and pattern?
|
inner and outer longitudinal, circular middle. Controlled by sympathetics in multiunit pattern.
|
|
|
As the ampulla of the vas deferens approaches the prostate, it is joined by the
|
seminal vesicle
|
|
|
True or False. The ejaculatory ducts have no muscle coat.
|
True
|
|
|
The power for ejaculation comes primarily from
|
smooth muscle of the vas deferens and caudal portion of the epididymis.
|
|
|
Accessory genital glands include
|
seminal vesicles, prostate gland, bulbourethral glands. All androgen dependent
|
|
|
True or False. Seminal vesicles are one continuous, highly folded, sheet of epithelium
|
True
|
|
|
Seminal vesicle epithelium is
|
pseudostratified columnar. Contains tall nonciliated (secretory) and short cells
|
|
|
The secretory product of the seminal vesicles constitutes
|
50-70% of the volume of semen.
|
|
|
Pale yellow color of semen is due to
|
flavins (lipochrome pigment). Causes an intense green fluorescence with UV light.
|
|
|
Seminal fluid contains
|
fructose (and other sugars), seminal vesicle-specific proteins, amino acids, ascorbic acid, and prostoglandins.
|
|
|
Prostate gland is derived from
|
endoderm
|
|
|
The formation, synthesis, and release of prostatic fluid is regulated by
|
dihydrotestosterone (5-alpha-reductase)
|
|
|
Prostatic secretions are rich in
|
zinc, citric acid
|
|
|
Prostatic epithelium also secretes
|
acid phosphatase, prostate-specific acid phosphatase (PAP), prostate-specific antigen (PSA), fibrinolysin
|
|
|
Typical prostatic cancer is in the
|
main gland
|
|
|
Bulbourethral glands are known as the
|
Cowper's glands
|
|
|
Cowper's glands secrete
|
galactose, galactosamine, galacturonic acid, sialic acid, and methylpentose
Responsible for preseminal fluid |
|
|
Root of the penis consists of
|
two crura, bulb, and associated muscles
|
|
|
Body of the penis consists of
|
two dorsal masses of erectile tissue (corpora cavernose) and a ventral mass of erectile tissue surrounding the penile urethra(corpus spongiosum)
|
|
|
Corpus spongiosum ends distally in the
|
glans penis
|
|
|
Dense fibroelastic layer that binds the three vacernosa together
|
tunica algubinea
|
|
|
Foreskin is known as the
|
prepuce
|
|
|
Erectile tissues of the corpora cavernosa receive blood from branches of the
|
dorsal and deep arteries of the penis
|
|
|
Venous drainage occurs by the
|
deep dorsal vein
|
|
|
When the penis is flaccid, much of the arterial blood flow is diverted into
|
AV anastomoses that connect the branches of the deep arteries of the penis to veins that deliver their blood into the deep dorsal vein.
|
|
|
In the FLACCID STATE, the AV SHUNT is
|
OPEN (blood flow bypasses the vascular spaces of the erectile tissue)
|
|
|
The shift in blood flow that leads to erection is controlled by the
|
parasympathetics
|
|
|
The parasympathetic impulses trigger local release of ... which cases relaxation of smooth muscles of the branches of the deep and dorsal arteries, increasing blood flow into the organ.
|
nitric oxide. Simultaneously, the AV anastomoses undergo construction, diverting the blood flow into the helicine arteries.
|
|
|
During erection, the AV SHUNT is
|
CLOSED (helicine arteries dilate and blood flows into the cavernous spaces/
|
|
|
Two chemicals control erection
|
nitric oxide and phosphodiesterase
|
|
|
cGMP, produced in response to NO, causes
|
relaxation of the smooth muscle cell wall.
|
|
|
What enzyme is produced to destroy cGMP and terminated erection
|
phosphodiesterase
|
|
|
Emission is a sequential release of products into the urethra. The order of release...
|
1) Bulbourethral glands add lubricant
2) Prostate adds enzymes 3) Testicular ducts (tail of epididymis and vas deferens) add sperm 4) Seminal vesicles add nutrients |
|
|
Ejaculation is regulated by the
|
sympathetic nervous system
|
|
|
Impulses from the sympathetic nervous system trigger
|
1) contraction of the smooth muscles of the genital ducts and accessory glands forces the semen into the urethra
2) The sphincter muscle of the urinary bladder contracts, preventing the release of urine 3) The bulbospongiosus muscle, which surrounds the proximal end of the bulb of the penis, undergoes powerful, rhythmic contractions, resulting in forceful expulsion of semen from the urethra. |
|
|
Ejaculation is followed by
|
cessation of parasympathetic inpulses to the vascular supply of the penis.
|
|
|
Volume of ejaculate
|
3ml. 20% of sperm are morphologically abnormal, and nearly 25% are nonmotile.
|
|
|
sterile male has a sperm count of less than
|
20 million sperm/ml
|
|
|
sperm maturation happens in the
|
epididymis
|
|
|
sperm capacitation happens in the
|
female reproductive tract
|
|
|
Sperm released from the testis and entering the epididymal duct have circular motion. After a 2-week maturation process, sperm acquire
|
forward motility
|
|
|
Sperm head consists of three components
|
condensed nucleus, acrosome, plasma membrane
|
|
|
The acrosome consists of 3 things
|
outer acrosomal membrane, inner acrosomal membrane, hydrolytic enzymes (hyaluronidase and acrosin)
|
|
|
Three main events during fertilization
|
1) acrosomal reaction
2) sperm binding to ZP3 3) sperm-oocyte fusion |
|
|
Hyaluronidase dissolves
|
intercellular material between the cells of the corona radiata
|
|
|
Binding to ZP3 causes
|
release of acrosin
|
|
|
Acrosin facilitates
|
penetration of the zona by the sperm head
|
|
|
Sperm binding induces Ca+-dependent exocytosis of the
|
cortical granules
|
|
|
Plasma membrane fusion is induced by
|
disintegrin
|
|
|
Cortical reaction prevents
|
polyspermy
|
|
|
Fast component of cortical reaction
|
change in resting membrane potential of the oocyte (only lasts a few minutes)
|
|
|
Slow component of cortical reaction
|
release of contents of cortical granules into perivitelline space (hydrolyzes ZP3)
|
|
|
Entry of sperm nucleus triggers
|
secondary oocyte to resume second meiotic division (results in ovum and second polar body)
|
|
|
Femail pronucleus and male pronucleus fuse forming
|
zygote (46,2N)
|
|
|
Unfertilized egg lacks
|
centriole (provided by sperm)
|
|
|
Window of time between ovulation and fertilization is about
|
24 hours
|
|
|
Meiosis is termed
|
reduction division
|
|
|
The first meiotic division is characterized by
|
a prolonged prophase
|
|
|
During the first meiotic division the centromere does not
|
divide
|
|
|
During the second meiotic division the chromosomes become arranged in a
|
metaphase plate and the centromeres divide
|
|
|
In the male, 4 cells are produced from 2 meiotic divisions (all viable sperm). How many in the female?
|
3 cells, but only 1 viable egg
|
|
|
Stages of meiosis
|
Prophase I
Metaphase I Anaphase I Telophase I Prophase II Metaphase II Anaphase II Telophase II |
|
|
Stages of Prophase I (meiosis)
|
1) leptotene
2) zygotene 3) pachytene 4) diplotene 5) diakinesis "Let's Zip & Package Diploid DNA" |
|
|
In this stage, sister chromatids appear as single rather than double threads. The telomeres of the chromosomes are attached to the nuclear envelope
|
leptotene
|
|
|
In this phase, the homologous chromosomes pair (point-for-point correspondence along their length), process called synapsis, forming synaptonemal complex. Resulting structure of this phase is a tetrad.
|
zygotene
|
|
|
In this stage, chromosomes pack and genetic exchange, called crossing over, occurs between chromatids (can occur between sister and nonsister)
|
pachytene
|
|
|
In this stage, the synaptonemal complex disassembles and the bomologous chromosomes separate from each other except at two of more specific connecting sites call chiasmata (where crossing over occured)
|
diplotene
|
|
|
In oocytes, meiosis is arrested at this stage until puberty.
|
diplotene
|
|
|
This refers to suspended diplotene state
|
dictyotene stage
|
|
|
In this phase, sister chromatids have beens attached via their centromeres as well the chiasmata. The nuclear envelope disappears
|
diakinesis
|
|
|
In this stage, the bivalent chromosomes are arranged on an equitorial plate. Each centromere is attached to spindle fibers
|
Metaphase I
|
|
|
In this state, the chromosomes (2 chromatids each) move to opposite poles. The chiasmata separate.
|
Anaphase I
|
|
|
In this phase, dyads lie near the poles. Cytokinesis takes place. AKA reduction division because haploid number is reduced.
|
telophase
|
|
|
There is a short interphase between
|
first and second meiotic division, during which neither DNA synthesis nor centriole duplication takes place.
|
|
|
During this phase, nuclear envelope breaks down, chromosomes move equatorially. No crossing over!
|
Prophase II
|
|
|
Chromatids are aligned on the equatorial plate and centromeres device and attach to spindle fibers
|
metaphase II
|
|
|
Chromatids (daughter chromosomes) move to opposite poles
|
anaphase II
|
|
|
Nuclear envelope reforms, uncoiling of chromosomes, development of daughter cells
|
telophase II
|
|
|
Chromosome configuration through the stages of meiosis
|
46,2N->46,4N->23,2N->23,1N
|
|
|
Aneuploidy refers to
|
any deviation in the normal number of chromosomes (detected by karyotyping). Includes trisomy and monosomy.
|
|
|
Trisomy 21. Characterized by mental retardation, short stature, stubby appendages, congenital malformations.
|
Down's syndrome
|
|
|
Associated with aneuploidy of the sex chromosomes (XXY). Characterized by infertility, variable degrees of masculinization, and small testes.
|
Klinefelter's syndrome
|
|
|
Assiociated with monosomy of the sex chromosomes (XO). Characterized by short stature, sterility. Compatible with life in contrast to other types of monosomy, which are lethal.
|
Turner's syndrome
|

