![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
370 Cards in this Set
- Front
- Back
|
The epithelium at the ends of the GI tract have what type of cell structure?
|
Statified squamous
|
|
|
What cranial nerve innervates the gut tube?
|
Vagus (CN X)
|
|
|
What are the four layers of the GI tract as you move out from the lumen?
|
1. mucosa
2. submucosa 3. muscularis externa 4. adventitia or serosa (if associated with mesentary) |
|
|
What are the three layers of the mucosa and what do each contain?
|
1. epithelium - cells specialized for regional function
2. lamina propria - loose connective tissue with vasculature, lymphatics and defense cells 3. muscularis mucosa - smooth muscle to help fold the mucosa |
|
|
In what GI tract layer is the Meissner Plexus and what is contained there?
|
Submucosa
Contains few cell bodies of enteric nerves and nonmyelinated post-symp fibers and a few pre-para fibers |
|
|
Where is the Auerbach plexus found?
|
between the circular and longitudinal muscles of the muscularis external
|
|
|
What are the 5 types of cells in the epithelium of the stomach lumen and gastric glands?
|
1. Mucus cells
2. Parietal cells 3. Chief cells 4. Stem cells 5. Endocrine cells |
|
|
What is the function of parietal cells in the stomach?
|
1. secrete HCl
2. produce intrinsic factor that binds to B12 in small intestine, promoting reabsorption |
|
|
What is the main function of Chief cells in the stomach?
|
produces pepsinogen, which is converted to active pepsin at high stomach pH
|
|
|
What three structures contribute to the 600-fold increase in surface area of the small intestine?
|
1. plicae circulares (spirally arranged folds)
2. villi (cover the plicae) 3. microvilli |
|
|
Where are the crypts of Lieberkuhn?
|
at the base of the villi and are continuous with the epithelium covering the villi
|
|
|
What is the cell type of the epithelium of the small intestine mucosal layer?
|
simple columnar
|
|
|
What are the 5 cells of the small intestine mucosal epithelium?
|
1. enterocytes
2. goblet cells 3. paneth cells 4. endocrine cells 5. stem cells |
|
|
What is the role of paneth cells in the small intestine?
|
defensive cells at base of crypts; phagocytose bacteria and protozoa and synthesize and secrete lysozyme and defensins
|
|
|
What hormones are produced by small intestine endocrine cells?
|
Cholocystokinin (CCK) and secretin
|
|
|
What are the three specialized cells in the submucosa of the small intestine and what do they do?
|
1. Brunner's Glands (duodenum) - secrete neutral to alkaline mucus to neutralize chyme
2. Peyer's Patches (ileum) - non-encapsulated lymphoid nodes 3. M cells - phagocytose lumenal contents and present antigens to underlying lymphocytes or macrophages |
|
|
What are two distinctive features regarding large intestine histology?
|
No Paneth Cells
No digestive enzymes in the apical plasma membrane |
|
|
How is the pacreatic gland organized cellularly?
|
gland is organized into acini arranged at the end of a common duct
|
|
|
What is the distinguishing feature of the gallbladder's mucosal layer?
|
There is no defined muscularis mucosa
|
|
|
How does ACh cause smooth muscle to contract in the GI tract?
|
causes action potentials to fire at each peak and the smooth muscle contracts at the frequency set by the BER
|
|
|
What is segmentation?
|
An isolated uncoordinated contraction propels contents in both directions, when the contraction relaxes, the contents go back to their original place -- mixing has occured without net propulsion
|
|
|
What is peristalsis?
|
coordination of proximal and distal GI tract segment contractions results in the net propulsion of contents
|
|
|
At what point is swallowing all reflex (involuntary)?
|
when the nasopharynx is closed off by the upward movement of the soft palate and the contraction of the upper constrictor muscle
|
|
|
What is receptive relaxation?
|
the process by which the stomach accomodates large volumes of contents (up to 1.5 L) without a marked increased in intra-gastric pressure.
|
|
|
How is receptive relaxation in the stomach controlled?
|
CN X
|
|
|
What hormone increases peristaltic contractions and decreases pyloric tone in the stomach?
|
Gastrin; release in response to food in stomach.
|
|
|
How are food contents moved through the small intestine?
|
Segementation and peristalsis
|
|
|
What is the function of the mirating myoelectric motor complexes (MMC)?
|
To remove bacteria and undigestible materials from the gastric atrium and small intestines
|
|
|
When does the MMC occur?
|
Only during fasting and terminates during eating
|
|
|
What hormone initiates the process of MMC?
|
motilin released from the small intestine
|
|
|
What type of movement is unique to the colon?
|
Mass movement or giant migrating contractions
|
|
|
How is HCl secreted by parietal cells?
|
1. active transport across apical membrane by H/K ATPase
2. HCO3 transported across the basolateral membrane in exchange for Cl by Cl/HCO3 anion exchanger (2' active transport) 3. Cl accumulates in the cell and is transported across the apical membrane by facilitated diffusion 4. H2O follows Cl out of cell down osmotic gradient |
|
|
What 3 substances initiate the secretion of acid?
|
ACh, gastrin, and histamine
|
|
|
What are the two routes by which ACh, gastrin and histamine potentiate release of HCl from parietal cells?
|
1. Direct pathway - ACh, gastrin and histamine directly stimulate the parietal cell
2. Indirect pathway - ACh and gastrin stimulate the enterochromaffin-like cell (ECL) resulting in the secretion of histamine. Then histamine acts on the parietal cell. |
|
|
What are the four phases of acid secretion?
|
1. Basal phase (interdigestive)
2. cephalic phase 3. gastric phase 4. intestinal |
|
|
Which phase of acid production accounts for the greatest amount of acid produced?
|
Gastric phase (50-60%)
|
|
|
What two factors protect the gastric mucosa from acid?
|
Unstirred mucosal barrier and bicarb secretion
|
|
|
Amylase catalyzes the hydrolysis of which glucose polymer?
|
the alpha-1,4 (it cannot lyse 1,6)
FREE GLUCOSE IS NEVER THE PRODUCT OF AMYLASE |
|
|
What is the name of the brush-border transporter of glucose, galactose and Na?
|
SGLT-1 (sodium dependent glucose transporter)
|
|
|
What is the name of the baso-lateral enterocyte transporter of glucose, galactose and fructose?
|
GLUT2 (sodium independent fructose transporter)
|
|
|
What causes lactose intolerance?
|
absence of the brush border enzyme lactase
|
|
|
What is the pathophysiology behind osmotic diarrhea in lactose intolerance?
|
unabsorbed lactose draws water into the intestinal lumen
|
|
|
What disease is caused by the genetic absence of Na/glucose cotransporter SGLT1 in the intestinal brush border?
|
glucose-galactose malabsorption
|
|
|
What are the endopeptidases associated with protein digestion?
|
1. Pepsin
2. trypsin 3. chymotrypsin 4. elastase |
|
|
For the following enzyme state (1)which organ secretes it, (2)what it digests and in what organ, (3)and what is the end product:
Amylase |
1. salivary glands
2. starch in the mouth and stomach 3. polysaccharides |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Lingual Lipase |
1. serous glands of the tongue
2. fat in the mouth and stomach 3. monoglycerides and fatty acids |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Trypsin,Chymotrypsin, Elastase, Carboxypeptidase |
1. Pancreas
2. Proteins and polypeptides in the duodemun and jejunum 3. small peptides and amino acids |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Amylase |
1. pancreas
2. starch in the duodenum and jejunum 3. polysaccharides |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Lipase-colipase, phospholipase A2, non-specific lipase |
1. pancreas
2. fat in the duodenum and jejunum 3. monoglycerides, fatty acids and cholesterol |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Sucrase-isomaltase |
1. brush border of the small inestine enterocytes
2. breaks 1,6 linkages of glucose (starch) in the intestine 3. glucose |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Maltase-glucoamylase (MGA) |
1. brush border enterocytes (sm intest)
2. maltotriose and maltose in the intestine 3. Glucose |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Lactase |
1. brush border enterocytes in sm intestine
2. lactose in the small intestine 3. glucose and galactose |
|
|
For the following enzyme state which organ secretes it, what it digests and in what organ, and what is the end product:
Sucrase |
1. brush border enterocyte in the sm intestine
2. sucrose in the small intestine 3. glucose and fructose |
|
|
Which intestinal sugar transporters are responsible for bringing glucose, glactose and fructose across the apical membrane fromt he lumen?
|
SGLT1 and GLUT5
|
|
|
Which intestinal sugar transporters bring glucose, galactose and fructose across the basolateral membrane into the blood?
|
GLUT2
|
|
|
At what end of the protein do exopeptidases hydrolyze amino acids?
|
At the C terminus
|
|
|
What proteases exist at the brush border?
|
aminopeptidase
dipeptidyl aminopeptidase dipeptidase |
|
|
At what end of the protein do proteases remove aminio acids?
|
the N-terminus (amino)
|
|
|
What cells in the stomach secrete pepsinogen?
|
Gastric chief cells
|
|
|
What is the basic mechanism for protein absorption across the enterocyte apical membrane?
|
Na-dependent cotransport
|
|
|
What are the differences in molecular absorption between carbohydrates and proteins?
|
Carbohydrates - only monomers are absorbed
Proteins - di- and tripeptides are absorbed intact |
|
|
What is the pathophysiology of Cysteinuria?
|
genetic absence/defect of the Na amino acid transporters (also in kidneys) resulting in the excretion of amino acids in the feces
|
|
|
What is the pathophysiology of Hartnup disease?
|
Genetic absence/defect of the neutral amino acid transporter in enterocytes
|
|
|
What are the steps onvolved in the digestion and absorption of lipids?
|
1. triglycerides are emulsified by bile salts and lecithin (increses surface area)
2. digested by lipase and colipase 3. solubilized in bile-salt micelles 4. passive diffusion fromt he membrane into the enterocyte 5. re-synthesis of triglycerides from monoglycerides and fatty acids 6. packaged into chylomicrons 7. incorporated into secretory vessicles in the Golgi 8. exocytosis into the ISF 9. enter lacteals and are taken to the thoracic duct and gen circulation |
|
|
What is the effect of aldosterone on the colon?
|
Colonic epithelial cells are signalled to absorb more Na and subsequently more H2O
|
|
|
How are water soluble vitamins absorbed?
|
B,C,niacin, folic acid, pantothenic acid and biotin are absoorbed either by cotransport with Na or passive diffusion
|
|
|
What disease is caused by impaired absorption of Vitamin B12?
|
Pernicious anemia
|
|
|
Describe the pathophysiology of achalasia.
|
degeneration of neural plexi within the musculature of the esophageal wall causing the LES to have incresed tone leading to difficulty swallowing
|
|
|
What pts are most likely to suffer from Mallory-Weiss tears?
|
EtOHics
|
|
|
Where are the 2 common sites of pill esophagitis?
|
1. site where aortic arch compresses the esophagus
2. LES |
|
|
What is shown on biospy of pill esophagitis?
|
Mucosal necrosis from high concentrations of drug in direct contact with mucosal surface
|
|
|
What is the most common cause of chemical esophagitis?
|
Reflux esophagitis
|
|
|
Mild to moderate esophagitis shows what on biopsy?
|
1. inflammatory cells infiltrating the mucosa
2. elongation and vascular congestion of papillary extensions of the LP |
|
|
What is the histological appearance of a Candida infection (fungal) of the esophagus?
|
1. exudates overlying shallow erosions of squamous epithelium
2. yeast ("stones") and pseudohyphal ("sticks") forms |
|
|
Malignant degeneration in Barrett's esophagus is only associated with which type of metaplasia?
|
Intestinal metaplasia (specialized columnar epithelium resembling intestinal mucosa)
|
|
|
What is the classic histological marker of intestinal metaplasia?
|
Goblet cells
|
|
|
What type of cancer is associated with Barrett's esophagus?
|
adenocarcinoma is strongly associated with GERD and Barrett's
|
|
|
What is the difference between an erosion and an ulcer?
|
Erosion - shallow lesion destroying the superficial aspect of the mucosa
Ulcer - mucosa is completely destroyed through underlying layers of bowel wall |
|
|
What are the hallmark characteristics of acute gastritis?
|
multiple, small, shallow erosions in the mucosa
|
|
|
What are the two types of disitnguishable chronic gastritis?
|
Active and autoimmune
|
|
|
What are the histologic signs of chronic gastritis?
|
infiltration of the lp of the gastric mucosa with plasma cells (density of plasma cells labels it as mild, moderate, and severe)
|
|
|
What characterizes active gastritis?
|
The presence of PMN within the gastric epithelium (if only in the lp, it is not active gastritis); mucosal atrophy
|
|
|
What is the eventual outcome of autoimmune gastritis?
|
complete destruction of parietal cells and megaloblastic anemia (do to lack of intrinsic factor --> B12 def)
|
|
|
What is the most common form of chronic gastritis?
|
H. pylori (esp, chronic active gastritis)
|
|
|
What two characteristics allow H pylori to survive in the stomach?
|
Urease - cleaves urea to make NH3; microenvironment with higher pH than gastric mucus
Adhesins - allows H pylori to adhere to the surface of gastric epithelial cells |
|
|
What test is used to differentiate malignant gastric carcinoma from benign peptic ulceration?
|
endoscopic biopsy
|
|
|
What is the cause of hypertrophic parietal cells in Zollinger-Ellison syndrome?
|
high serum gastrin levels as a result of non-regulated gastrin secretion from a neuroendocrine tumor.
|
|
|
What is the cause of hypertrophic parietal cells in hypertrophic-hyperceretory gastropathy?
|
idiopathic; no high serum gastrin levels
|
|
|
What is the risk of peptic ulcer disease in pts with Menetrier Disease?
|
there is no risk of PUD since acid production is not increased
|
|
|
What are the 2 major histological patterns of small intestinal diseases?
|
1. villous blunting with or without lymphocytosis of the superficial epithelium (e.g. gluten enteropathy)
2. distention of the small intestinal villi (Whipple Disease and Mycobacterium avium intracellulare (MAI) |
|
|
Where are the majority of gastrointestinal stromal tumors (GISTs) located?
|
Stomach (50-60%)
|
|
|
What nerve innervates striated and smooth muscle of the esophagus to facilitate swallowing?
|
CN X
|
|
|
What is the primary clinical feature of achalasia?
|
dysphagia for both solids and liquids
|
|
|
What is the classical radiographic presentation of achalagia?
|
the bird's beak-like narrowing at the esophagogastric jnxn
|
|
|
What is the underlying disease process in the esophagus from scleroderma?
|
vasuclar obliteration and fibrosis in gut smooth muscle --> wekaend the LES --> GERD
|
|
|
What are the three factors necessary to see the development of GERD?
|
1. reflux
2. refluxed material must be caustic to the mucosa 3. casutic material must have a sufficient duration of contact with the esophageal mucosa |
|
|
What are the sequelae to prolonged GERD?
|
1. erosions/ulcers
2. scarring/benign stricture 3. re-epithelialization with squamous cells - Barrett's |
|
|
What are the characteristics of Distinctive-type Barrett's esophagus?
|
gastric-type columnar epithelium with intestinal metaplasia (presence of goblet cells)
|
|
|
What is the other term for "Steak house syndrome"
|
Schatzki rings.
|
|
|
Pseudomembranous exudate of C. difficile colitis
|
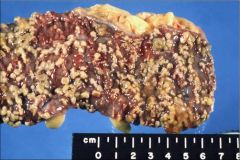
Describe the appearance of this colon segment. What is the pathology?
|
|
|
Amebiasis abscesses (can develop at distal sites such as liver, inguinal lymph nodes and the brain)
|
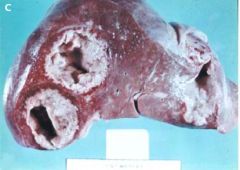
This picture shows the invasion of what pathology to the liver?
|
|
|
diverticulosis due to lack of fiber in his diet
|
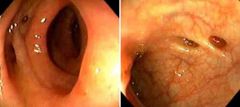
An 85 year old man presents to the ER with LLQ tenderness to deep palpation and alternating bouts of constipation and diarrhea. A scope reveals the image above. What is the cause of this man's symptoms?
|
|
|
Chronic inactive colitis:
1. decreased number of crypts 2. shortened crypts 3. architectual distortion |
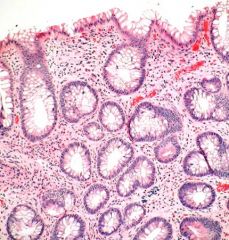
Describe the histological features of this slide. What is the pathology?
|
|
|
Creeping fat adherening to the bowel wall. Crohn's disease.
|
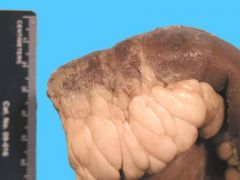
Describe the appearance of this bowel segment. What is the pathology?
|
|
|
deep fissure with abundant lymhocytes (blue patches between mucosa and submucosa) = Crohn's
|
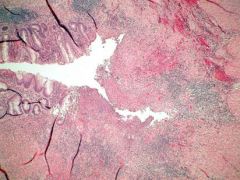
Describe the histo-pathology present in this slide. what disease process is present here?
|
|
|
This is ulcerative colitis:
1. toxic megacolon (exaggeration of the colon to the point of focal necrosis and possible rupture) 2. adenocarcinoma |
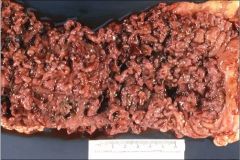
What are the complications resulting from the disease shown in this photo?
|
|
|
This is melanosis coli, typically caused by laxative abuse
|
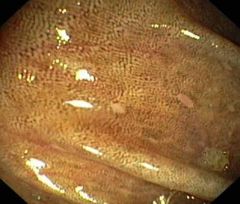
A 22-year-old bulimarexic visits her PCP complaining of chronic diarrhea. Endoscope reveals the image above. what is the cause of this pts diarrhea?
|
|
|
This is gastric metaplasia of the esophagus. GERD is the most common cause and inappropriate relaxation of the LES appears to be the most important mechanism for GERD
|
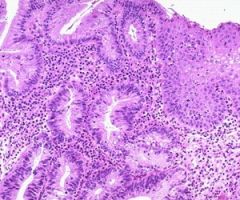
What is the pathophysiology most commonly associated with the presence of this histology?
|
|
|
What cause for dysphagia is most often associated with episodic food obstruction (asymptomatic between episodes)?
|
Webs or rings
|
|
|
What is odynophagia?
|
Pain with swallowing
|
|
|
From where do somatic efferent cholinergic fibers of the vagus nerve originate?
|
nucleus ambiguus
|
|
|
A patient with a 30 year history of uncontrolled GERD has the following scope. What two features are noticable?
|
Barrett's esophagus with adenocarcinoma
|
|
|
What feature is present in the smooth muscle of the esophagus in a pt with achalasia?
|
aperistalis
|
|
|
How does scleroderma cause esophageal injury?
|
vascular obliteration and fibrosis in gut smooth muscle weaken the LES and predispose the pt to GERD
|
|
|
Heamtemesis suggests a bleed in what part of the GI tract?
|
Upper GI between the mouth and distal duodenum
|
|
|
Melena suggests a bleed in what part of the GI tract?
|
distal small bowel and proximal colon
|
|
|
Autoimmune metaplastic atrophic gastritis is caused by what kind of antibodies?
|
Anti-parietal and anti-intrinsic factor anitbodies
|
|
|
What are the serum gastrin levels in a pt with autoimmune atrophic gastritis?
|
High. Since the antrum is spared, gastrin levels are elevated without the production of HCl
|
|
|
What are the 3 major causes of peptic ulcer disease?
|
1. gastric acid (like in Z-E)
2. h. pylori 3. NSAIDS |
|
|
What causes refractory duodenal ulcers in Z-E syndrome?
|
non-physiological gastric acid hypersecretion due to gastrin-secreting islet cell tumor of the pancreas.
|
|
|
What must the serum gastrin levels be to consider a dx of Z-E?
|
> 1000pg/ml
|
|
|
What percentage of pts with gastrinomas will have metastises at the time of clincal presentation?
|
70-80%
|
|
|
What are the 3 stages that result in h. pylori gastritis?
|
1. recruitment of inflammatory cells
2. activation of inflammatory cells 3. epithelial damage by cell activity |
|
|
Which cytokine is released in response to h. pylori antigen exposure?
|
IL-8
|
|
|
What are the 2 types of epithelial damage caused by h. pylori?
|
1. direct damage by adherence of h. pylori to mucosal wall = loss of microvolli and cell injury
2. damage to cell mucosa as a consequence of the recruited inflammatory cells. |
|
|
What is the most specific test for h. pylori? Cheapest? Good but expensive?
|
Specific = stool antigen test (but false negatives if on PPIs)
Cheapest = serum tests Good/expensive = breath urease tests |
|
|
At low saliva flow rates, what is the lumenal concentrations of HCO3 and Cl?
|
At low flwo rates, there is time for ductal cells to modify secretion so there is more HCO3 in the lumen and Na and Cl move out - very hypotonic
|
|
|
How are pancreatic zymogens actived in the small intestine?
|
chyme arrives in small intestine --> release CCK -> stimulate acinar cell secretion through IP3/ca 2nd msgrs -> zymogens secreted into small intestine -> trypsin activated by small-intestine produced enterokinase -> trypsin cleaves/activates the rest of the enzymes
|
|
|
What is the mechanism for the neutralization of chyme in the duodenum?
|
chyme arrives in small intestine -> presence of acid causes secretion of secretin from duodenal cells -> secretin stimulates pancreatic duct cells to secrete bicarb-rich fluid -> bicarb neutralizes acid in small intestine
|
|
|
What is the primary stimulant of enzyme secretion from acinar cells?
|
CCK
|
|
|
What is the structural defect associated with infantile hypertrophic pyloric stenosis (IHPS)?
|
hypertrophy and hyperplasia of the smooth muscle in the gastric wall at the level of the pylorus that leads to a narrowing of the antrum and near obstruction
|
|
|
What is the clinical presentation of infantile hypertrophic pyloric stenosis (IHPS)?
|
projectile vomiting starting at 3 weeks of age
|
|
|
What is the prominent feature of Hirschprung's Disease?
|
Megacolon: massive dilitation of the intestinal lumen
|
|
|
What is the pathophysiology of Hirschprung's Disease?
|
genetic mutation causing a failure of the Auerbach and Meissner plexi to form in a segment of hte bowel wall -> absence of ganglion cells
|
|
|
What type of diarrhea is associated with toxin-producing bacteria?
|
explosive, onset soon after exposure
|
|
|
What organisms are associated with toxin-causing intestinal infections?
|
C. perfringens, botulinum, E. coli, Vibrio cholerae, C. diff, Campylobacter
|
|
|
Invasive bacteria cause what type of diarrhea?
|
exudative diarrhea that is usually bloody and marked abdominal pain
|
|
|
Bacterial enterocolitis shows what type of histological pattern?
|
active colitis: exudate, PMNs and little destruction of the colonic architecture
|
|
|
Which viral enterocolitis is most common in children?
|
Rotavirus
|
|
|
Why is appendicitis a surgical emergency?
|
If inflammatory reactions weaken the bowel wall perforation can occur, spilling fecal material and bacteria into the peritoneal cavity
|
|
|
What are the three most important differentiating facts between Crohn's and Ulcerative colitis?
|
1. UC - continuous involvement of the mucosa/ CD - skip lesions
2. UC - inflammation limited to mucosa and submucosa/ CD - transmural lymphoid aggregates 3. UC - toxic megacolon/ CD - perforations and fistulas |
|
|
Between CD and UC which is more likely to develop adenocarcinoma?
|
both are equally as likely to develop adenocarcinoma
|
|
|
What are the two main pathophysiological mechanisms for diarrhea?
|
1. decreased absorption (osmotic)
2. increased secretion (secretory) |
|
|
What three mechanisms account for diarrhea due to decreased absorption?
|
1. inhibitied or defective absorptive processes (e.g., E. coli)
2. osmotically active agents in the lumen 3. increased motor activity |
|
|
What are the two main mechanisms responsible for diarrhea caused by increased secretion?
|
1. activated intestinal secretion of Cl and/or HCO3 (water passively follows)
2. increase in crypt cells mass or crypt hyperplasia ->increased anion secretion (e.g. celiac sprue) |
|
|
What are the common causes of water diarrhea of an osmotic variety?
|
1. carbohydrate malabsorption
2. osmotic laxatives |
|
|
What are the common causes of water diarrhea of a secretory variety?
|
1. bacterial toxins
2. neuroendocrine tumors 3. ileal bile salt malabsorption 4. disorders with a dysmotility component |
|
|
What is the specific membrane receptor to which cholera toxin binds resulting in the activation of adenylate cyclase?
|
GM 1 ganglioside on the luminal membrane of enterocytes
|
|
|
How does heat-stable E. coli cause diarrhea?
|
stimulates guanylate cyclase and increase cyclic GMP
|
|
|
Where is the only site of active bile acid absorption from the intestine?
|
ileum
|
|
|
What drug can be given orally to bile-acid induced diarrhea associated with limited resection or disease of the terminal ileum?
|
Cholestyramine
|
|
|
Diabetic diarrhea is caused by what process?
|
abnorlam motility
|
|
|
What are the main differences between secretory and osmotic diarrhea?
|
Secretory - large volume, persists during fasting, "osmotic gap" < 50
Osmotic - volume less than 1L/day, decreases or ceases during fasting, "osmotic gap" > 125 |
|
|
How do you calculate the osmotic gap?
|
2 x ([Na] + [K])=290
|
|
|
What are the common causes of fatty diarrhea?
|
malabsorption syndromes (celiac, Whipple's, short-bowel syndrome) and maldigestion syndromes (pancreatic insufficiency, inadequate luminal bile salts, biliary obstruction)
|
|
|
What are the three mechanisms that Celiac sprue causes diarrhea?
|
1. decreased brush border hydrolase activity
2. villus atrophy -> loss of surface area -> nutrient malabsorption 3. crypt hyperplasia -> electrolyte hypersecretion |
|
|
What are the mucosal pathologies of untreated celiac sprue?
|
severe villous atrophy, crypt hyperplasia, enterocyte disarray and inflammatory infiltrate of the lp and epithelium
|
|
|
What is the function of the right colon?
|
absorption of water and ions
|
|
|
What is the main function of the transverse, descending and signmoid colon?
|
store waste and indigestible materials before elimination
|
|
|
severe case of celiac sprue; loss of villi, flattening of the mucosa (may also see crypt hyperplasia, not in this slide)
|
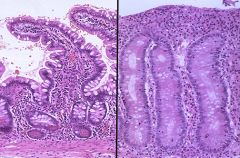
Normal intestingal mucosa is on the left. Describe the intestinal muscoa on the R and name the disease.
|
|
|
Obstruction from the exophitic nature of adenocarcinomas of the colon.
|

What is a presenting symptom of the disease represented in the above photo?
|
|
|
serositis, paralytic ileus, and toxic megacolon are the most dangerous acute complications of what disease?
|
ulcerative colitis
|
|
|
Omeprazole and Lansoprazole are not as effective against what type of GI disturbance?
|
They are PPI's; since not all pumps are active at the same time, it may take several days to reach maximal effectiveness = not good for acute gastritis (use H2 antagonists instead)
|
|
|
for whom should you adjust the dosing protocol when using Ranitidine?
|
It is a H2 receptor antagonist that has renal elimination; dosage reduction required if impaired renal fnxn/elderly
|
|
|
Which H2 antagonist has CYP450 metabolism, increasing the toxicity of other drugs?
|
Cimetidine
|
|
|
Which drug is typically used as an adjunct to peptic ulcer therapy due to its ability to form a protective barrier over ulcerative tissue?
|
Sucralfate (a mucosal protective agent)
|
|
|
Misoprostol is only indicated for what type of ulcers?
|
NSAID-induced ulcers
|
|
|
What is one of the possible consequences of antacids?
|
Rebound acid secretion if the pH is raised to 7
|
|
|
Which antacids cause constipation?
|
Calcium and aluminum
|
|
|
Which antacids cause diarrhea?
|
Magnesiun ("Oh My God, i pooped my pants)
|
|
|
What is the MOA of gastrointestinal motility agent, Metoclopramide)?
|
Dopamine antagonist that blocks presynaptic inhibition of ACh release
|
|
|
Which drug is currently restricted to the treatment of severe IBS in women with diarrhea as the prominent symptom?
|
Alosteron, a serotonin 5-HT4 antagonist
|
|
|
Which drug is now restricted to women under 55 with IBS: constipation predominent or chronic idiopathic constipation?
|
Tegaserod (constipation gives me "gas")
|
|
|
Which two classes of drugs are typically used as anti-emetic agents?
|
Serotonin (5HT) receptor antagonists and Dopamine receptor antagonists
|
|
|
Ondansetron (a 5HT receptor antagonist) prevents vomiting due to what causes?
|
cytoxic drugs and post-op nausea
|
|
|
Metoclopramide (a dopamine receptor antagonist) prevents nausea due to what cause?
|
Chemotherapy-induced vomiting
|
|
|
Which classes of drugs are commonly used to treat n/v caused by motion sickness?
|
Histamine and muscarinic receptor anagonists?
|
|
|
What is the MOA of bulk-forming laxatives?
|
stimulate peristalsis via absorption of water and bulk expansion
|
|
|
What are the 4 categories of Laxatives?
|
bulk-forming, saline (osmotic), stimulant (irritant), and wetting agents/stool softeners
|
|
|
Which drug is useful in both constipation and diarrhea?
|
Polycarbophil--it absorbs 60x its weight in water AND prevents fecal desiccation
|
|
|
Which anti-diarrheal should be avoided in children under 12 due to risk of Reye's syndrome?
|
Bismuth subsalicylate
|
|
|
What is the primary tx for severe diarrhea in compromised patients?
|
Oral rehydration therapy
|
|
|
hypertrophy of the muscular layer of the wall
presence of chronic inflammatory cells Rokitansky-Aschoff sinuses |
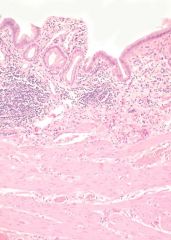
This is a histological slide of chronic cholecystitis. What patterns are noticible here?
|
|
|
Rokitansky-Aschoff sinus - a herniation of the gallbladder mucosa through the muscularis
|
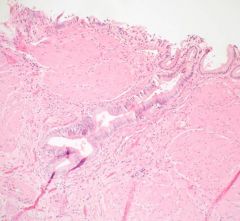
This is a slide of chronic cholecystitis. What is the identifying feature on this slide?
|
|
|
Which hormone triggers the contraction of the gallbladder after a fatty meal?
|
CCK
|
|
|
What are the risk factors for chololithiasis?
|
fat, forty, female, fertile
|
|
|
Where in the gallbladder do you typically find obstruction in acute calculous cholecystitis?
|
Neck
|
|
|
What is the most common predisposing factor for cholangiocarcinoma?
|
gallstones
|
|
|
What are the histologic findings of acute pancreatitis?
|
necrosis and peripancreatic fat
|
|
|
how do mutations in SPINK1 and PRSS1 lead to acute pancreatitis?
|
SPINK1 mutation = no inhibition of PRSS1
PRSS1 mutation = self activation of trypsin Both lead to increased trypsin activity > acinar cell damage > more enzymes released > increasing tissue damage... |
|
|
Histologically, what is seen with chronic pancreatitis?
|
fibrosis, sparing of islets until later in disease, proteinaceous plugs, pseudocysts (also in acute)
|
|
|
Why aren't pseudocysts real cysts
|
they lack and epithelial lining
|
|
|
What is the clinical presentaiton of pancreatic ductal carcinoma?
|
abdominal pain, weight loss, jaundice, malabsorption
|
|
|
Which pancreatic carcinomas are associated with Trousseau's syndrome?
|
this thromboembolic disorder is associated with mucin-producing pancreatic carcinomas
|
|
|
Cancers in what part of the pancreas are you more likely to see Trousseau's syndrome?
|
head and tail, where most mucin is produced
|
|
|
What other diseases are linked to Neuroendocrine tumors?
|
multiple endocrine neoplasia syndrome 1 (MEN1) and von Hippel Lindau syndrome
|
|
|
Which type of islet cell tumor is associated with Zollinger-Ellison syndrome?
|
Gastrinoma
|
|
|
Which type of islet cell tumor presents with hypoglycemia?
|
insulinoma (tumor of beta cells)
|
|
|
What are the two mechanisms for the development of gallstones?
|
1. Cholesterol hypersecretion
2. Bile acid hyposecretion |
|
|
Black pigement stones are the result of what process?
|
Increased deconjugated bilirubin from GB stasis, infection, hemolysis and/or cirrhosis
|
|
|
Symptoms of acute cholangitis include Charcot's triad and Reynold's Pentad. What are these symptoms?
|
Charcot's triad - RUQ pain, jaundice, fever
Reynold's Pentad - all ofthe above plus hypotension and mental confusion |
|
|
What is the most common cause of adolscent jaundice?
|
Gilbert's syndrome
|
|
|
What are markers of chronic pancreatic damage?
|
fibrotic pancreas with calcium stones in the duct
|
|
|
What is the cause of systemic complications from Acute pancreatitis?
|
cytokines derived in the pancreas act directly on organ tissues and activate local inflammatory response --> cellular injury and organ dysfunction
|
|
|
What is the most common etiology of chronic pancreatitis?
|
alcohol abuse
|
|
|
A mutation in what gene is a predominant inherited condition that causes chronic pancreatitis?
|
CFTR (cystic fibrosis)
|
|
|
For pancreatitis:
Acute:necrosis::chronic:______ |
fibrosis
|
|
|
What cells in the liver play a central role in fibrosis?
|
Stellate cells - they turn into collagen-synthesizing cells when stimulated
|
|
|
Steatorrhea will not occur in a person with a normal diet until pancreatic lipase secretion decreases to what percentage of normal?
|
10% - steatorrhea doesn't appear until more than 90% of the gland has been destroyed
|
|
|
Wha tis the most frequent presenting symptom of chronic pancreatitis?
|
Pain that never entirely disappears; epigastric but may radiate to the back
|
|
|
What is the 5-year survival ratefor esophageal cancer?
|
5%
|
|
|
What type of gastric adenocarcinoma presents with signet ring cells and linitis plastica?
|
Diffuse type
|
|
|
What are the presenting symptoms of gastric cancer?
|
pain, early satiety/anorexia/weight loss
bleeding/anemia |
|
|
What is the long term survival from gastric cancer in the US?
|
< 10%
|
|
|
The majority of pancreatic tumors are of which type?
|
Adenocarcinomas (cancers of the exocrine pancreas)
|
|
|
Where is the most common location of pancreatic tumors?
|
Head
|
|
|
What are the risk factors for pancreatic cancer?
|
Smoker, diabetes, chronic pancreatitis, genetic factors
|
|
|
What is the median survival (in months)from pancreatic cancer without surgery? After surgery?
|
6 months without surgery
20 months with surgery |
|
|
Familial adenomatous polyposis is associated with a mutation of the APC gene at what locus?
|
5q21
|
|
|
What 2 syndromes correlate the presence of adenomatous polyposis with other lesions?
|
Gardener's Syndrome (desmoid tumors and osteomas)
Tucot's Syndrome (malignant brain tumors) |
|
|
Lynch Syndrome (aka HNPCC) is caused by a defect in which repair mechanism?
|
mismatch excision and repair of misincorporated DNA bases
|
|
|
What are the most commonly mutated genes in Lynch syndrome?
|
MSH2 and MLH1
|
|
|
What are the most common cause of rectal bleeding in children?
|
Juvenile polyps
|
|
|
What is the most common type of gastric polyp?
|
Fundic polyp
|
|
|
Which section of the colon accounts for the greatest % of colon carcinoma?
|
1. Descending and rectosigmoid colon (43%)
2. Ascending (38%) |
|
|
For colorectal cancer:
proximal tumors:______lesions::distal tumors:______lesions |
exophytic
circumferential |
|
|
Aggressive periodontis is characterized by which features?
|
rapid loss of dental attachment to bone and bone destruction
|
|
|
What is the smallest individual functional unit in the liver?
|
the lobule
|
|
|
What are the three types of lobules in the liver?
|
classic, portal and acinar
|
|
|
The classic lobule is organized in respect to what?
|
the flow and drainage of blood
|
|
|
What constitutes the portal triad?
|
interlobular branches of the portal vein, hepatic artery, and bile duct
|
|
|
The protal lobule is arranged with respect to what?
|
Exocrine function/bile secretion - triangle connecting three central veins with a portal triad at the center.
|
|
|
List the three mechanisms by which hepatic sinusoids create an large area for intestinal blood to contact hepatocytes?
|
1. portal system - capillary beds in the liver
2. minimizing structures between sinusoids and hepatocytes 3. arrangement of hepatocytes into plates one cell thick with at least two surfaces exposed to blood = maximal surface area |
|
|
What is the role of Kupfer cells in the sinusoid?
|
Resident macrophages to destroy bacteria from enteric venous blood
|
|
|
Under normal conditions, what is the role of stellate cells in the Space of Disse?
|
They take up and store fat and fat soluble vitamins like vitamin A
|
|
|
Outline the path of bile from the hepatocyte to the gallbladder?
|
hepatocyte secretes bile into bile canaliculi >portal triad >collecting bile ducts > hepatic duct > gallbladder
|
|
|
What may be some non-heptic reasons for an increase in aminotransferases?
|
drugs/medications
skeletal muscle (AST only) cardiac muscle/MI (AST only) |
|
|
Describe the difference in AST/ALT elevtaions in a patient with acute liver disease versus chronic liver disease
|
acute liver disease most often has marked elevated AST/ALT whereas chronic liver disease usually has a modest elevation (2-10 fold)
|
|
|
Alcoholic hepatitis usually shows an AST:ALT great than what?
|
> 2 (>1 is indicative of cirrhosis for any cause)
|
|
|
What are the causes of a very high (>10) ALT?
|
viral, drug, toxic, ischemic (alcohol shows lower ALT)
|
|
|
Nirtofuran causes what kind of hepatitis?
|
chronic active (will resove with discontinued use)
|
|
|
Cholestatic liver injury represents dysfuntion in what region of the hepato-biliary system?
|
the biliary tract (either intrahepatic or extrahepatic)
|
|
|
Which liver fnxn tests are used to diagnose cholestatic liver disease?
|
alk phos
GGT |
|
|
Hepatic parenchymal diseases (hepaocellular) are caused by what etiologies?
|
viral (HCV,HBV), autoimmune, NASH, genetic
|
|
|
In what other conditions may one see an increase in alk phos?
|
bone disorders
cancer pregnancy |
|
|
Will the GGT be elevated or normal in liver/billiary disease?
|
Elevated
|
|
|
What etiologies produce cholestatis?
|
gallstones
billiary tumors PSC - primary sclerosing cholangitis PBC |
|
|
Overproduction of bilirubin (hemolysis) creates an increase in what type of bilirubin in the blood (conjugated vs unconjugated)?
|
unconjugated
|
|
|
What genetic disorder causes a mild decrese in UGT activity and therefore a mild increase in unconjugated bilirubin in the blood?
|
Gilbert's Syndrome
|
|
|
What genetic disorder causes a major deficiency in UGT activity and therefore a marked increase in unconjugated bilirubin in the blood?
|
Crigler-Najjar
|
|
|
In Dubin-Johnson Syndrome, where is the metabolism deficiency?
|
Decrease activity of MRP2 (ABCC2) which transports bilirubin out of the hepatocyte (increased in blood conjugated bilirubin)
|
|
|
Cholestasis will result in an increased of which type of bilirubin?
|
Conjugated
|
|
|
In liver patients, decreased albumin is indicative of what disease?
|
cirrhosis and favors formation of ascites and edema
|
|
|
What are the normal lab values for AST/ALT?
|
both < 40 U/L
|
|
|
What are the normal lab values for Alk Phos?
|
< 120 U/L
|
|
|
What are the normal lab values for total bilirubin? conjugated (direct)?
|
< 1.0 mg/dL
direct , 0.2 mg/dL |
|
|
What are the normal lab values for prothrombin time?
|
< 12 sec
|
|
|
What are the normal lab values for Albumin?
|
> 3.8
|
|
|
What are the criteria for fulminant hepatic failure?
|
progression to liver failure within 4 weeks of the onset of illness
|
|
|
Fulminant hepatic failure is primarily doe to what cause?
|
drug toxicity, specifically acetaminophen toxicity
|
|
|
What is the treatment for a patient with acetaminophen toxicity?
|
N-acetyl-cysteine
|
|
|
Outline the progression of necrosis in acute hepatitis?
|
diffuse inflammation and disorganized architecture > mild necrosis around terminal hepatic venules > bridging necrosis > submassive necrosis (only small islands of periportal hepatocytes viable) > massive necrosis (no viable tissue remains)
|
|
|
What is the route of infection for pts with acute hepatitis from HAV?
|
Fecal-oral route; usually from contaminated food (produce) or water
|
|
|
What are two specific histological findings of an HBV infection?
|
ground-glass hepatocytes (viral particle inclusion in a crystaline array within the cytoplasm of the hepatocyte)
sanded nuclei (smudgy eosinophilic inclusions in the nuclei of hepatocytes) |
|
|
Which hepatitis syndrome is more common with HCV, acute or chronic?
|
Chronic; HCV accounts for 70% of chronic hepatits cases and almost never causes acute hepatitis syndrome
|
|
|
Reaction to which medication is a causative factor in NASH?
|
Amiodarone - a strong anti-arrhythmic
|
|
|
What is the mechanism of primary billiary cirrhosis (PBC)?
|
autoimmune; anti-mitochondrial antibodies (AMA)
|
|
|
What other GI disease seems to be associated with primary sclerosing cholangitis (PSC)?
|
inflammatory bowel disease (at least 2/3 of patients with PSC have IBD)
|
|
|
What is the cause of secondary sclerosing cholangitis?
|
obstruciton of the bile ducts > inflammatory reaction > periportal fibrosis > cirrhosis
|
|
|
secondary sclerosing cholangitis
|
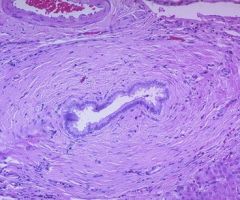
Onion skin fibrosis, shown here, is the classic hisotlogic lesion of what nonviral liver pathology?
|
|
|
Hemochromatosis has what etiology?
|
Iron overload from AR genetic mutation in the HFE gene or from excessive iron intake (Bantu siderosis, repeated transfusions)
|
|
|
Which HFE mutation shows the more severe form of hemochromatosis?
|
C282Y homozygotes
|
|
|
Where else might one find iron deposition in a pt with hemochromatosis?
|
pancreas (leading to diabetes) and myocardium (conduction abnormalities and arrhythmias)
|
|
|
When does Wilson's disease typically present?
|
childhood (first or early second decade)
|
|
|
What is the cure for Alpha-1-antitrypsin deficiency?
|
liver transplant; since A1A is synthesized in the liver
|
|
|
Hepatocellular adenoma is thought to be related to what medication?
|
oral contraceptives
|
|
|
Where are frequent sites of metastacies for hepatocellular carcinoma?
|
lung, brain, bone marrow
|
|
|
Multiple billiary cysts can be present when the liver is involved in what other disease?
|
AD PKD
|
|
|
Where is a cholangiocarcinoma LEAST likely to appear?
|
in the common bile duct
|
|
|
What is the common macroscopic feature of cholangiocarcinoma?
|
Klatskin Tumor - hilar mass at the confluence of hepatic ducts
or peripheral mass in the liver |
|
|
What is the most common benign tumor of the liver?
|
hemangioma
|
|
|
What is the presentation of perinatal biliary atresia?
|
nml at birth with late onset jaundice and progressively acholic stools due to prgressive destruciton of the biliary tree
|
|
|
When should hepatoportoenterostomy (Kasai procedure) be performed to ensure greater success in treating biliary atresia?
|
before day of life 60
|
|
|
Which hereditary disorders lead to serum unconjugated hyperbilirubinemia?
|
Crigler-Najjar Syndrome and Gilbert's Syndrome
|
|
|
Which hereditary disorders lead to serum conjugated hyperbilirubinemia?
|
Dubin-Johnson Syndrome
Rotor syndrome |
|
|
What is the classic PE sign of wilson's disease?
|
Kayser Fleischer rings in the corneas
|
|
|
What are the two most common intrinsic hepatotoxins and what form of toxicity do they cause?
|
Acetaminophen --> hepatocellular necrosis
Amiodarone --> heatic steatosis |
|
|
At 24 year old female visits here PCP for a bladder infection. She is given antibiotics. After two weeks she presents to the ER with RUQ pain and AST/ALT elevations. A liver biopsy shows hepatocellular necrosis. What is the cause of her hepatocellular injury?
|
Autoimmune reaction to the hepatocyte membrane from nitrofurantoin (antibiotic used for UTI)
|
|
|
What are the most common patterns of hepatotoxicity?
|
hepatocellular, cholestatic, mixed
|
|
|
Fasting and Gilbert's Syndrome inhibit what factor in the acetaminophen metabolism pathway?
|
Glucuronyl transferase is inhibited forcing the molecule down the CYP450 pathway
|
|
|
Chronic alcoholism has what affect on the acetamminophen metabolism pathway?
|
induces the CYP450 and decreases glutathione which increases acetaminophen-derived hepatotoxins
|
|
|
Hepatocellular injury commonly shows what patterns in lab values?
|
elevated AST and ALT with ALT > 2x upper limit of normal
again, associated with acetaminophen, statins, isoniazid, cloxacillin |
|
|
Cholestatis injury commonly shows what patterns in lab values?
|
Alk Phos > 2x upper limit of nml
|
|
|
Mixed injury commonly shows what patterns in lab values?
|
ALT/ULN:AP/ULN ration between 2 and 5
ALT not more than 5x more abnl than alk phos caused by anabolic steroids, tamoxifen, estrogens |
|
|
What diseases are commonly associated with macrovesicular steatohepatitis?
|
alcoholism, diabetes, obesity, metabolic syndrome
|
|
|
What diseases are commonly associated with microvesicular steatohepatitis?
|
mitochondrial dysfunction
acute fatty liver of pregnancy Reye syndrome Valproic acid, tetracycline |
|
|
Name the two clinical syndromes associated with cirrhosis?
|
portal hypertension and liver insufficiency
|
|
|
Portal hypertension and liver insufficiency result in what signs, respectively?
|
Portal htn: ascities and varicies
Liver insufficiency: jaundice |
|
|
What are the physical signs and lab tests that help diagnose cirrhosis?
|
PE: palpable L lobe with a small R lobe (on percussion) and splenomegally
Labs: serum albumin and prolonged PT, hyperbilirubinemia, low platelet count |
|
|
Where is the site of increased resistance in portal HTN?
|
intra-hepatic/sinusoidal
|
|
|
Variceal hemorrhage is more likely with which clinical features?
|
large variceal size (increased pressure on the wall) Child B/C score and red whale markings on the varicies (indication of prior bleeding)
|
|
|
What must the HVPG (hepatic venous pressure gradient) be above for ascities to occur? To what SAAG level does this score correspond?
|
HVPG: 11-12 mmHg
SAAG: 1.1 g/dL |
|
|
What other test must be performed to distinguish intrahepatic (cirrhosis) from posthepatic (Budd-Chiari) causes of ascities?
|
Ascites total protein content
> 2.5g/dL = peritoneal causes |
|
|
What is the mechanism behind the development of hepatorenal syndrome?
|
extreme peripheral vasodilation resulting in renal vasoconstriction
|
|
|
What is the difference between Type 1 and Type 2 heaptorenal syndrome?
|
Type 1 - rapid renal failure (doubling of serum creatinine)
Type 2 - slowly progressing, result of refractory ascities |
|
|
What is the only definitive treatment for HRS?
|
liver transplant
|
|
|
What is the mechanism behind hepatic encephalopathy?
|
hepatocellular damage > unable to metabolize ammonia adequately > substances alter the stucture/function of brain cells (astrocytes)
|
|
|
How does lactulose work to decrease ammonia in hepatic encephalopathy?
|
1. acidic pH decreases urease-producing bacteria which produce ammonia
2. extra proton combines with NH3 to form NH4 which is non-absorbable --> ammonia excreted in the stool |
|
|
What is the mechanism for the development of portopulmonary HTN?
|
pulmonary bed exposed to vasoactive substances produced in splanchnic circulation and bypassed liver metabolsim --> endothelial proliferation, vasoconstriction, in situ thrombosis, and obliteration of vessels
|
|
|
What are the signs of pulmonary HTN?
|
dyspnea, syncope, chest pain, accentuated second heart sound and R ventricular heave
|
|
|
What must a pts Child score be to be placed on the liver transplant list?
|
7 (of 15)
|
|
|
What pts have the highest priority on the liver trnasplant list?
|
Pts with fulminant hepatic failure
|
|
|
QUESTIONS
|
ANSWERS
|
|
|
What properties of chest pain suggest GERD?
|
Burning, antacids help, NO exercise correlation/shortness of breath
|
|
|
What should GERD patients avoid eating?
|
High-fat foods, chocolate, peppermint
|
|
|
Alcohol and Tobacco cause what 3 esophogeal Sx?
|
LES Relaxation, Impaired Tissue resistance, reduced acid clearance
|
|
|
When should PPIs be taken for maximum effectiveness?
|
30-60 minutes before eating
|
|
|
What stain is used to confirm presence of Goblet cells in the esophagus?
|
Alcian blue (pH 2.5 mucin stain)
|
|
|
If a GERD patient with Barrett's esophagus suddenly presents with Dysphagia, what is your concern?
|
Adenoma has developed (or less serious, a stricture)
|
|
|
What are the primary Sx of B12 deficiency?
|
Megaloblastic anemia, parasthesias, irritability, memory impairment, psychosis/depression
|
|
|
List the THREE causes of B12 deficiency?
|
Poor Diet, malabsorption, Intrinsic factor deficiency
|
|
|
Which B12 deficiency cause indicates Pernicious Anemia?
|
Intrinsic factor deficiency
|
|
|
What gastric cell type secrets Intrinsic Factor (and is absent in Pernicious Anemia Patients)?
|
Parietal Cell
|
|
|
What Intestinal Cell Type, when present in the gastric mucosa, indicates a progression towards gastric carcinoma?
|
Goblet Cells
|
|
|
What gastric cell type proliferates in the prescence of prolonged, elevated pH?
|
ECL Cells (gastric body), G cells (gastric antrum)
|
|
|
Do carcinoid tumors or adenocarcinomas of the stomach have greater malignant potential?
|
Adenocarcinoma
|
|
|
What are two possible causes of gastric outlet obstruction?
|
malignancy (adenocarcinoma or lymphoma) or Ulcer (causes fibrosis/scarring of Gastroeduodenal anastamosis)
|
|
|
Chronic emesis can cause what serum level abnormality?
|
metabolic alkalosis (from vomiting up HCl)
|
|
|
Name three types of Bezoars
|
phytobezoar (vegatable matter), trichobezoar (hair), pharmacobezour (pills)
|
|
|
The transmural inflammation seen in Crohn's disease leads to what serious complication?
|
fistula and sinus tract formation
|
|
|
Granulomas are seen in what inflammatory bowel condition?
|
Crohn's disease
|
|
|
When do we order fecal leukoctyes?
|
to differtiate inflammatory from non-inflammatory diarrhea
|
|
|
Evaluation of chronic, non-bloody diarhea involves what stool study?
|
Stool electrolytes
|
|
|
Name three causes of bloody diarrhea?
|
The Three I's: Infection, Ischemia, Inflammation
|
|
|
What is the difference between a true and false diverticulum?
|
Trues have all intestinal wall layers represented
|
|
|
Name THREE effects of EtOH on the pancreas:
|
Ischemia, decreased protein secretion, toxicity (via metabolites), elevated CCK, cytokines
|
|
|
Define saponification.
|
precipitation of calcium salts within areas of necrotic adipose tissue (literally, "soap-making")
|
|
|
How much pancreatic function can be lost before becoming symptomatic?
|
90% (usually lipase goes first - so steatoffhea presents first)
|
|
|
Describe TWO mechanisms for an increased INR.
|
1) Decreased Liver synthetic function 2) malabsorption of fat soluble vitamins (due to pancreatic disease)
|
|
|
What is the prognosis of insulinoma?
|
Good - usually benign and only 10% are multifocal
|
|
|
What is the difference between cholelithiasis and cholecystitis?
|
cholelithiasis is the presence of gall stones, whereas cholecystitis is the inflammation that occurs when the gall stones obstruct the cystic duct.
|
|
|
What is the difference between choledocholithiasis and cholangitis?
|
first is stones in the common bile duct, second is the bacterial infection that can result
|
|
|
What are the top risk factors for gallstones?
|
The four F's: Fat, Female, Fertile, and Forty
|
|
|
Intercellular bridges (desmosomes) are a feature of what GI neoplasm?
|
squamous cell carcinoma
|
|
|
What are signet-ring cells?
|
Individual, infiltrating cells with large mucin vaculous found in gastric adenocarcinoma
|
|
|
What tumor type is positive for CD117 staining, and what does it mean for Tx?
|
GIST - will be responsive to Gleevec
|
|
|
What THREE neoplasms can give rise to adenocarcinoma at the ampulla of Vader?
|
Villous adenocarcinoma (from bowel surface), pancreatic ductal adenocarcinoma, cholangiocarcinoma
|
|
|
What are the FOUR major patterns of liver disease?
|
Hepatocellular, Cholestatic, Synthetic, Infiltrative
|
|
|
AST/ALT only are elevated in what type of liver disease?
|
Hepatocelluar (non-liver: AST only could be skeletal/cardiac muscle)
|
|
|
What lab values are elevated in cholestatic liver disease?
|
AP, GGT, and Bilirubin (non-liver: AP w/o GGT elevation could be bone disease)
|
|
|
What lab test is the best indicator for decreased hepatic synthetic function?
|
INR (non-liver: could be vitamin K deficiency or inherited coagulopathy if albumin is normal)
|
|
|
If ALP (aka Alk. Phos for you Tommy Bang fans out there) is the only elevated lab value, what pattern of liver disease is likely?
|
Infiltrative (Non-liver: lack of GGT elevation indicates bone disease)
|
|
|
MASSIVE (>15xULN) AST/ALT elevations can be cause by what?
|
viral infection, autoimmune hepatitis, ischemia (budd chiari/shock liver), drugs/toxins (NOT alcohol alone)
|
|
|
Which Immunoglobulin sub-type must be present to indicate an ACTIVE infection?
|
IgM (IgG only indicates past exposure)
|
|
|
Which type of hepatitis is transmitted by the fecal-oral route?
|
Hep A (B and C are blood-borne)
|
|
|
What histologic abnormalities are seen in Hep B infection?
|
ground glass inclusions (from virus particles) and sanded nucleus
|
|
|
Hep C infection is found in what percentage of chronic alcoholics?
|
up to 50%
|
|
|
What liver injury cause is likely if AST>ALT elvevation? Vice-versa?
|
alchohol; viral disease/toxins
|
|
|
Name FOUR common dietary deficiencies found in chronic alcholics:
|
Water soluble Vitamins(Vit B series, C); Fat-soluble Vitamins(Vit a, D, K); Trace elements (iron, zinc, etc); Macronutrients (protein, fat, carbs)
|
|
|
What lab findings support cirrhosis?
|
low platelets, albumin, serum sodium
|
|
|
Portal hypertension due to cirrhosis will have what SAAG score?
|
>1.1g/dl (lower is tumor, intraperitoneal infeciton, other)
|
|
|
What are the three classifications of portal hypertension?
|
Prehaepatic (portal thrombosis), Intra heptatic (cirrhosis), Posthepatic (budd-chiari - hepatic ven thrombosis)
|
|
|
What are the three classifications of intrahepatic portal hypertension?
|
presinusoidal (schistosomiasis), sinusoidal cirrhosis), postsinusoidal (veno-occlusive disease)
|
|
|
What is hepatorenal syndrome?
|
renal failure in the setting of cirrhosis and absence of other types of renal disease
|
|
|
Name THREE complications of portal hypertension:
|
Hepatic encephalopathy, gastropathy, varices
|
|
|
Name the SIX triggers of hepatic encephalopathy in chronic liver disease:
|
azotemia, sedation from meds, GI Bleed, infection, volume depletion, excess protein
|
|
|
Primary Biliary Cirrhosis (PBC) is more common in which gender?
|
Women (>85%); other one's the other one: Men are ~70% cases of PSC
|
|
|
What lab pattern is seen with PBC and PSC?
|
Elevated alk phos (3-4x), bili high in PSC from beginning, PBC later in course
|
|
|
How is a PBC diagnosis made?
|
Antimitochondrial antibodies (AMA)
|
|
|
How is a PSC diagnosis made?
|
Cholangiography (ERCP, MRCP, PTC) - beads on a string appearance from saccular dilations in hepatic ducts
|

