![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Features of cancer |
Tissue invasion and metastasis
Sustained angiogenesis Self-sufficiency in growth signals Insensitivity to antigrowth signals Evasion of apoptosis Limitless replicative potential |
|
|
Structure |
Introduction
Before metastasis Cascade Epithelial-mesenchymal transition MMPs degrade ECM Cell motility regulation Colonisation Lymphatic vessels Conclusion |
|
|
Introduction Localised tumours, current science, where do they mainly occur? |
Primary, localised tumours are responsible for about 10% of cancer deaths.
Majority of deaths caused by cancer are due to metastasis. Many aspects of invasion and metastasis formation are still poorly understood · Arrest in target organs · 80% of life-threatening cancers occur inepithelial tissues – carcinomas |
|
|
Before cascade Angiogenesis What is required for cascade? |
· Carcinoma cells often succeed in stimulatingangiogenesis on stromal side of membrane. · Invasion through BM places them in a better position to execute invasion- metastasis cascade |
|
|
Two major events in metastasis? |
- Physical translocation of cancer cells from theprimary tumour to a distant organ - Colonisation of the translocated cells within thatorgan. |
|
|
Events in cascade |
Phenotype change Local invasion intravasation: Circulation: Extravasation. Immune system evasion: Arrest in target organs Colonisation: |
|
|
Phenotype change |
Cancer cells acquire within primary tumour acquire an invasive phenotype |
|
|
What is loss of basal lamina directly correlated with? |
probability of developing distantmetastasis |
|
|
Local invasion: changes required advantage What is secreted? |
Disruption of BM and Cell detachment(changes in cell-cell & cell-matrix contacts). Once present in stromal compartment, carcinoma cells gain direct access to blood and lymphatic vessels –nutrients and oxygen. Depends on release of secreted proteased to remodel ECM.Some invading carcinoma cells make their own proteases, MMP-2 and MMP-9. |
|
|
Intravasation: depends on what? |
Invade into the surrounding matrix and toward blood vessels - intravasate to enter the circulation. Depends on the ability of individual cancer cells or smallclumps of these cells to break away from their neoplastic neighbours and enter on their own into thecirculation. |
|
|
What are cancer cells in blood called? |
Circulating tumour cells |
|
|
Circulation: Properties displayed Why may they die? |
Display properties of anchorage-independentsurvival. May die by apoptosis triggered by detachment of a cell from solidsubstance |
|
|
Extravasation. Why might they die? |
In the distant organ, CTCs exit the circulation and invade into the microenvironment of the foreign tissue. Blood –hydrodynamic shear forces which are substantial in smaller vessels – tear cellsapart. |
|
|
Immune system: |
At that foreign site, cancer cells must be able to evade the innate immune response and also survive as a single cell (or as a small cluster of cells). |
|
|
Arrest in target organs: What is required? |
To develop into an active macrometastatic deposit, the cancer cell must be able to adapt to the micro-environment and initiate proliferation |
|
|
Colonisation: |
Recruitment of new blood vessels |
|
|
Transition in cell type? Reason Associated loss? |
Epithelial-mesenchymal transition and associated loss of E-cadherin expression enable carcinoma cellsto become invasive
|
|
|
What is lost in EMT? |
tight junctions involving E-cadherin, epithelial cell polarity, gene expression program |
|
|
What is gained in EMT? |
fibroblast-like shape, motility, invasiveness, increased resistanceto apoptosis, mesenchymal; gene expression Tfs. Induced expression of vimentin– an intermediate filament component of mesenchymal cell cytoskeleton. Proteasesecretion – MMP-2 and MMP-9. Replacement of E- to N-cadherin – induces |
|
|
Reversibility of EMT? |
carcinomaoften revert back to a more epithelial phenotype by passing through METtransition (opposite) |
|
|
Control of EMT? |
TGF-B results in loss of epithelial morphology and reduction of epithelial markers |
|
|
EMT diagram |
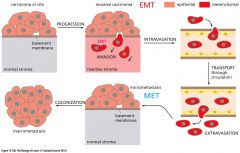
|
|
|
E to M transition diagram |

|
|
|
Diagram for stages of migration |
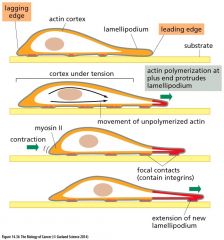
|
|
|
Different modes of migration of cancer cells |
Individual (mesenchymal) Collective (either as a wide front ornarrow string of cells Amoeboid |
|
|
Stages of migration |
· The cell organizes actin fibers inorder to extend lamellipodia at its advancing/leading edge and to establish newfocal contacts. · At the same time, stress fibers, alsoconsisting of actin, are used to contract the trailing edge of the cell, wherefocal contacts are being broken. · The making and breaking of the focalcontacts depend on localized modulation of the affinities of various integrinsfor extracellular matrix (ECM) |
|
|
How is cell motility regulated?
|
small G proteins of Rho family |
|
|
Diagram for regulation of motility |
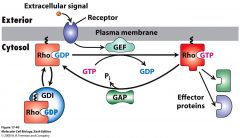
|
|
|
Explain motility regulation |
· GTPase regulate actin organization. Active when GTP bound. GEF mediate signal-induced switch to active state by accelerating release of GDP · GAP- enhances rate of GTP hydrolysis – inactives protein. GDI – retains Rho proteins in an inactive state in cytosol |
|
|
Contribution of Rho proteins in cell migration |
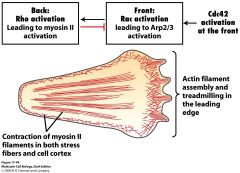
|
|
|
Role of MMPs in cell migration and cancel cell invasion? |
degrade ECM for migration
•Common theme: expressed as an inactive proenzyme · Clean a path through matrix · Expose cryptic sites on cleaved proteins· Promotion of cell detachment · Release signal proteins that stimulate cell migration Other roles: - angiogenesis, wound healing, |
|
|
How are MMPs controlled? |
•TIMPs = tissue inhibitors of MMPs •Local activation •Confinement by cell-surface receptors |
|
|
How are MMPs activated? What expresses MMPs? |
Proteolytic activation expressed by both the tumour cells and surrounding stromal cells |
|
|
Diagram showing Adaptation of metastatic cells to a foreign environment. |
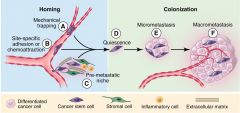
|
|
|
Stages above |
Homing - mechanical trapping, site-specific adhesion or chemoattraction, pre-metastatic niche Colonisation - quiescence, micro-metastasis, macro-metastasis |
|
|
What type of vessels are used to disperse cells from primary tumour? Importance for diagnosis |
lymphatic vessels · Prognostic importance of lymph node involvement in TNM classification. |
|
|
Colonisation steps |
· Micrometastases(detectable only by microscopy methods) – they can stay dormant for longperiods of time. · Formany cancers, the final step – growth of micrometastasis to macroscopic metastasis (colonisation) is a rate-limiting step. |
|
|
Portal circulation and liver metastases: |
venous blood from gut and spleen goes directly to liver through portal system Metastatic cells from colorectal cancers are therefore easily trapped in liver capillaries |
|
|
A model for acquisition of the colonising ability |
Genetically heterogenous primary tumour Dissemination of metastatic cells, subsequent removal of primary tumour Micrometastases scattered Acquisition of ability to colonise Macroscopic metastasis New, secondary shower of metastatic dissemination New colonisation - rapid expansion Multiple macroscopic metastases. |
|
|
Conclusion Invasion–metastasis cascade Frequency of success What is the least efficient step? |
involves local invasion, intravasation,transport, extravasation, formation of micrometastases, and colonization. Thesequence of steps in this cascade is completed only infrequently, resulting in metastaticinefficiency. The least efficient of these steps appears to be colonization. |
|
|
Conclusion EMT: When is it normally used? What does it enable? |
Many of these steps can be executed by carcinoma cells that activate a cell-biological program called the epithelial–mesenchymal transition (EMT),which is normally used by cells early in embryogenesis and during wound healing. The EMT involves loss of an epithelial cell gene expression program andacquisition of mesenchymal gene expression. The latter enables cells to acquireinvasiveness, motility, and a heightened resistance to apoptosis |
|
|
Conclusion Cell motility: How is it regulated? |
regulated by a series of small G proteins of the Rho familythat are activated by cytoplasmic signal-transducing pathways and control theassembly of the actin cytoskeleton |
|
|
Conclusion Cell invasiveness: What enables it? How are they made? |
enabled by various matrix metalloproteinases (MMPs) that functionto degrade components of the extracellular matrix. These enzymes are often manufactured by inflammatory cells within the tumor-associated stroma |
|
|
Conclusion Genotype changes: Are the changes major? |
The acquisition of invasive and metastatic powers does not appear to involve major changes in the genotype of cancer cells within the primary tumor. |

