![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
18 Cards in this Set
- Front
- Back
|
What is the rate of a chemical reaction |
How fast reactants are turned into products. |
|
|
Explain graphs for rate of reaction. |

They show how fast reactants are used up or products formed over time. The steeper the line and the quicker it gets flat show how fast the reaction is. |
|
|
What is a successful collision with particles |
A collision that ends in the particles reacting to form products. |
|
|
Activation energy |
The least amount of energy needed for particles to react |
|
|
What is a gas,solid and time measured in. |
Gas-cm^3 Solid- grams(g) Reactants could be moles (mol) |
|
|
Rate of reaction equation, how do you find a particular time aswell? |

|
|
|
What are the three ways to measure rate of reaction |

|
|
|
Name specific experiment which reacts to create gas |
Magnesium and HCL produce H2 gas
|
|
|
Specific experiment to create cloudy precipitate (yellow sulfur) |

Sodium thiosulfate+HCL |
|
|
How do you find mean rates of reaction from a graph |
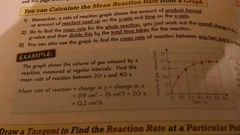
Change in y on graph/change in X Amount of reactants used or product formed/time |
|
|
How do you use a tangent to find rate of reaction at a specific point |
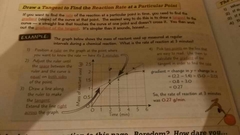
Create the tangent and work with that as the new line |
|
|
What is equilibrium |
With reversible reactions. When the reactants keep making products eventually they will slow down(forwards reaction) as there is less reactants. As there is more products they will react together, untill there is less products(backwards reaction). This keeps happening untill forwards and backwards reaction occur at the same rate. This is equilibrium. |
|
|
What happens if the position of the equilibrium is on the right |
There is more products (the right side) then reactants |
|
|
If a reaction is endothermic in one direction.. |
It is exothermic in the opposite direction |
|
|
What is le chateliers principle |
The idea if you change the conditions of a reversible reaction the system will try to counteract that change |
|
|
What happens if you change the temp in a reversible reaction |
If you decrease the temp, the reaction would move in the exothermic direction. This means you would get more products from the exothermic reaction and less from the endothermic reaction. The opposite happens if you increase the temperature. |
|
|
What if you increase the pressure in a reversible reaction? |
This only affect equilibrium involving gasses. If you increase the pressure the reaction moves in the direction were there is less molecules. You should check and balance equations. |
|
|
What if you increase the concentration in a reversible reaction? |
The system will no longer be in equilibrium... Remember this is the case as equilibrium is in a closed system were nothing comes out or in.if you increase the reactants the system makes more products . If you increase the products the system increases the amount of reactants It does this to bring it back to equilibrium. |

