![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
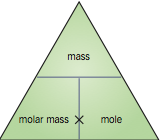
How to calculate Molar Mass |
|
|
|
How to calculate percentage masses |
1. Calculate formula mass 2. Multiply RAM by number of atoms 3. Divide certain element by total formula Mr times by 100 |
|
|
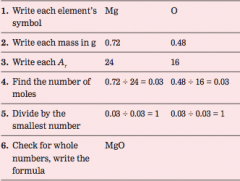
How to calculate empirical formula |
|
|
|
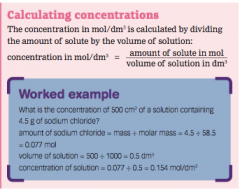
Calculating concentrations |
|
|
|
Volume of water to add for dilution |

|
|
|
Titration equations |
Convert volumes to dm^3 Unknown concentration = known concentration × volume of known / volume of unknown |
|
|
How to measure mass of gases |
Gas syringe, upward displacement and a balance |
|
|
Calculating moles of gas |
Volume divided by 24 |
|
|
How do equilibrium work |
Reversable reaction in a closed system Rate of forward reaction must stay equal to rate of backwards reaction If temperature is increased reaction moves towards endothermic If pressure is increased reaction moves towards the side with fewer molecules or moles |
|
|
Contact process |
1. Sulfur + oxygen - sulfur dioxide 2. Sulfur dioxide + oxygen - sulfur trioxide 3. Sulfur trioxide + water - sulfuric acid Optimum temperature of 450 Small pressure as high pressure isn't cost efficient V2O5 catalyst increases rate of reaction but doesn't effect equilibrium position |
|
|
Difference between weak and strong acids |
strong acid is fully ionised (in water) weak acid is only partially ionised |
|
|
What is a spectator ion |
Ions that don't take part in the reaction |

