![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is an Atom? |
The smallest part of a substance which can have its own characteristic properties. |
|
|
|
What is an Ion? |
A charged atom formed by loss or gain of an electron. |
|
|
|
What is an Isotope? |
Atoms of the same element that have a different number of neutrons and the same number of protons. |
|
|
|
What is an element? |
A pure substance made up of only one type of atom. |
|
|
|
What is a compound? |
A pure substance formed by chemically combining at least two different elements. |
|
|
|
What is a mixture? |
Materials made up of at least two substances which may be element or compound. |
|
|
|
What is an electron? |
A sub-atomic particle held by the positive protons in orbits called levels or energy. |
|
|
|
What is a proton? |
A sub-atomic particle with a positive charge found in the nucleus of the atom. |
|
|
|
What is a neutron? |
A sub-atomic particle that is found in the nucleus of an atom and has no electric charge. |
|
|
|
What is the relative atomic mas (ram) or (mass number) |
Protons +neutrons |
|
|
|
What is the atomic number? |
Protons or electrons. |
|
|
|
In the periodic table what are the rows called? |
Periods |
Across. |
|
|
In the periodic table what are the group 1's called? |
Alkali metals |
|
|
|
In the periodic table what is the middle section called? |
Transition metals |
|
|
|
In the periodic table what are the group 7's called? |
Halogens |
|
|
|
What is the atomic shell structure rules |
First shell 2 electrons Second shell 8 electrons Third shell 8 electrons Fourth shell 16 electrons |
|
|
|
What is the atomic structure of sulphur? |
2,8,6 |
|
|
|
How do you draw an isotope? |

|
|
|
|
How do you draw ionic bonding? |
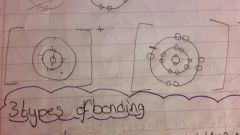
Above |
|
|
|
What are the 3 types of bonding. What are they between? |
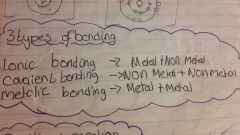
|
|
|
|
What is a positive ion called? |
Cation |
|
|
|
What is a negative ion called? |
Anions |
|
|
|
When do atoms become ions? |
When electrons are lost or gained. |
|
|
|
What is the charge of an atom when it loses an electron? |
Positive |
|
|
|
What is the charge of an atom when it gains an electron? |
Negative. |
|
|
|
What does covalent bonding look like? |
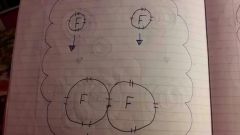
|
|
|
|
What are properties of covalent boning? (2) |
Share electrons Full outer shell. |
|
|
|
What do alkali metals react with? (2) |
Water Hologens (group 7) |
|
|
|
What is thermal decomposition? |
Break down with heat. |
|
|
|
What are properties of halogens? |

|
|
|
|
What are halogens used for? Why? |
Cleaning as they are posisonus. |
|
|
|
What does oilrig stand for? |
Oxidation Is Loss Reduction Is Gain |
|
|
|
What is the balanced equation for group 1 and group 7 reacting together? |
2 group1 + group7 2-> 2 g1g7 |
|
|
|
What are the electrons like in metallic bonding? |

|
|
|
|
In the periodic table what are the columns called? |
Groups |
Down |

