![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
4 Cards in this Set
- Front
- Back
|
What simple compounds will be formed from the following given ions: Li+F
Ba2+F
Al3+F |
Simple Compounds: LiF
BaF2
AlF3 |
|
|
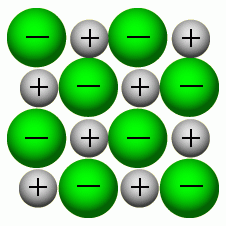
Describe the detail of an ionic lattice.
|
Positive and negative ions are joined together, forming a giant ionic lattice. Each one is held to the other by strong electrostatic forces of attraction. |
|
|
Explain properties of an ionic lattice. |
The ions being held together increases the boiling and melting point because it takes more energy to break the bonds. They are solid because the particles are packed tightly together. |
|
|
Giant Ionic Lattice: Sodium and Chlorine |
|

