![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
What is an element? |
An element is a substance mad of only one type of atom |
|
|
Where are protons, neutrons and electrons positioned in an atom? |
Protons - nucleus Neutrons - nucleus Electrons - outer shells what |
|
|
What are the relative charges of a Proton, a Neutron and a Electron? |
Proton - +1 Electron - -1 Neutron - 0 |
|
|
What are protons, neutrons and electrons called? |
Sub-atomic particles |
|
|
What are protons, neutrons and electrons called? |
Sub-atomic particles |
|
|
How do atoms have no overall charge? |
The number of electrons are equal to the number of protons and therefore the charges balance out, meaning there is no overall charge. |
|
|
What is the atomic number of a atom? |
The number of protons in an atom |
|
|
What is atomic mass? |
The sum of protons and neutrons in an atom |
|
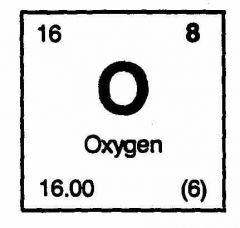
What are the number of protons,neutrons and electrons in oxygen? |
Protons - 8 Electrons - 8 Neutrons - 16-8 = 8 |
|
|
What is the rule for electronic structures of atom diagrams? |
Fill the inner shell first in a 2,8,8,2 order |
|
|
What are the group 1 metals called? How many electrons are in the router shell? |
- Alkali metals - 1 electron in their outer shell |
|
|
When alkali metals react with water what do they produce? |
A metal hydroxide + hydrogen |
|
|
When alkali metals react with water what do they produce? |
A metal hydroxide + hydrogen |
|
|
How do alkali metals react with oxygen? |
They burn vigorously when heated with oxygen |
|
|
When alkali metals react with water what do they produce? |
A metal hydroxide + hydrogen |
|
|
How do alkali metals react with oxygen? |
They burn vigorously when heated with oxygen |
|
|
What do alkali metals produce when they react with oxygen? |
Metal oxides |
|
|
What are the group 0 elements called? |
The noble gases |
|
|
Why are the noble gases unreactive? |
They have full outer shells/stable arrangements of electrons |
|
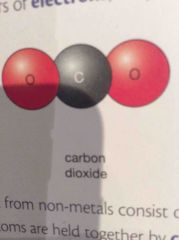
When atoms share pairs of electrons what type of bond is it? |
A covalent bond (Molecules) |

